
 Copy to clipboard
Copy to clipboard 
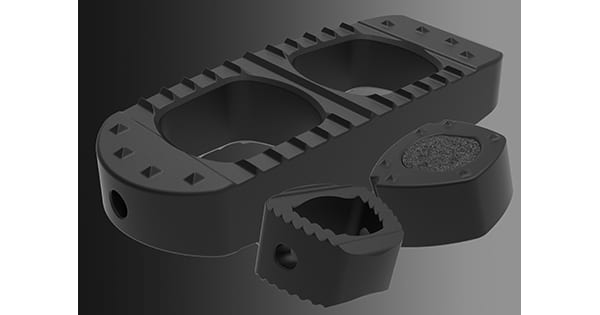
The Taiwan Food and Drug Administration granted CTL Amedica a license for the silicon nitride-based spinal platform of the Valeo II Lumbar Interbody Fusion Device, Valeo C+CSC Cervical Interbody Fusion Device and the Valeo II Cervical Interbody Fusion Device.
The product line is projected to launch in late 2020 in the region through distributor partner Watson Biomedical Technology.
“We began laying the groundwork for entrance into this important, thriving market upon participating in the 14th Annual Meeting of the Taiwan Neurosurgical Society in 2019, and we are pleased to see these efforts coming to fruition,” said Daniel Chon, CTL Amedica Corporation President and CEO. “The Valeo product line features our proprietary silicon nitride spine technology – the ideal biomaterial for fusion.”
The Taiwan Food and Drug Administration granted CTL Amedica a license for the silicon nitride-based spinal platform of the Valeo II Lumbar Interbody Fusion Device, Valeo C+CSC Cervical Interbody Fusion Device and the Valeo II Cervical Interbody Fusion Device.
The product line is projected to launch in late 2020 in the region through...
The Taiwan Food and Drug Administration granted CTL Amedica a license for the silicon nitride-based spinal platform of the Valeo II Lumbar Interbody Fusion Device, Valeo C+CSC Cervical Interbody Fusion Device and the Valeo II Cervical Interbody Fusion Device.
The product line is projected to launch in late 2020 in the region through distributor partner Watson Biomedical Technology.
“We began laying the groundwork for entrance into this important, thriving market upon participating in the 14th Annual Meeting of the Taiwan Neurosurgical Society in 2019, and we are pleased to see these efforts coming to fruition,” said Daniel Chon, CTL Amedica Corporation President and CEO. “The Valeo product line features our proprietary silicon nitride spine technology – the ideal biomaterial for fusion.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







