
 Copy to clipboard
Copy to clipboard 
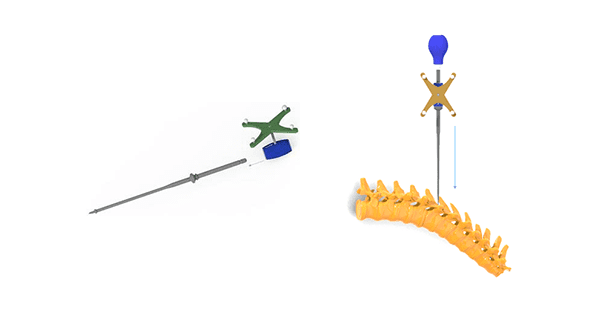
CTL Amedica received FDA 510(k) clearance to market the CTL Amedica Navigation Instrument System, featuring manual surgical instruments adaptable for use with third-party navigation, that are designed to assist surgeons in locating anatomical structures in either open, minimally invasive or percutaneous procedures for preparation and placement of pedicle screws.
CTL Amedica Navigation Instruments are manufactured from stainless steel, and the system is compatible with CTL Amedica’s RAPHAEL™ Pedicle Screw System Family, the PICASSO II™ MIS Spinal System Family and the TAURUS™ Pedicle Screw System Family.
The third-party navigation systems are indicated for any medical condition in which the use of stereotactic surgery may be appropriate and where reference to a rigid anatomical structure, such as vertebra, can be identified relative to a CT- or MR-based model, fluoroscopy images or digitized landmarks for the anatomy. The surgical imaging technology provides surgeons visualization for complex and MIS procedures and aids in establishing trajectory during advanced surgical procedures. These navigation systems provide surgeons with access to real-time, multi-plane 2D and 3D images, providing visual representation of hardware placement.
Source: CTL Amedica
CTL Amedica received FDA 510(k) clearance to market the CTL Amedica Navigation Instrument System, featuring manual surgical instruments adaptable for use with third-party navigation, that are designed to assist surgeons in locating anatomical structures in either open, minimally invasive or percutaneous procedures for preparation and placement of...
CTL Amedica received FDA 510(k) clearance to market the CTL Amedica Navigation Instrument System, featuring manual surgical instruments adaptable for use with third-party navigation, that are designed to assist surgeons in locating anatomical structures in either open, minimally invasive or percutaneous procedures for preparation and placement of pedicle screws.
CTL Amedica Navigation Instruments are manufactured from stainless steel, and the system is compatible with CTL Amedica’s RAPHAEL™ Pedicle Screw System Family, the PICASSO II™ MIS Spinal System Family and the TAURUS™ Pedicle Screw System Family.
The third-party navigation systems are indicated for any medical condition in which the use of stereotactic surgery may be appropriate and where reference to a rigid anatomical structure, such as vertebra, can be identified relative to a CT- or MR-based model, fluoroscopy images or digitized landmarks for the anatomy. The surgical imaging technology provides surgeons visualization for complex and MIS procedures and aids in establishing trajectory during advanced surgical procedures. These navigation systems provide surgeons with access to real-time, multi-plane 2D and 3D images, providing visual representation of hardware placement.
Source: CTL Amedica

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







