
 Copy to clipboard
Copy to clipboard 
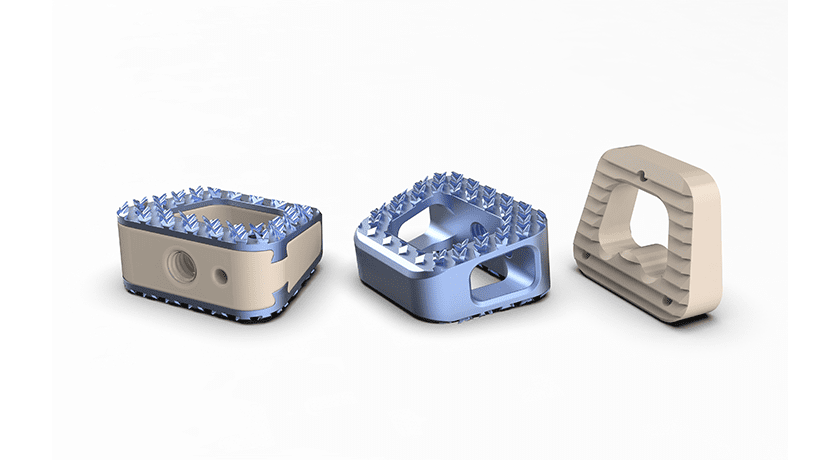
The Taiwan Food and Drug Administration (TFDA) granted a license to CTL Amedica for the MATISSE™ Anterior Cervical Interbody Fusion (ACIF) Cage. The official license, DHA 05603556709, enables CTL Amedica to market the MATISSE ACIF Cage in Taiwan.
The MATISSE ACIF Cage is engineered to accommodate a wide range of patient anatomies and surgeon preferences. It features streamlined instrumentation and a tapered leading edge for easy insertion, as well as a large graft area to further promote bone fusion.
The system is available in numerous footprints, heights, lordotic angles and materials, including titanium, PEEK and titanium-plated PEEK. Both titanium and titanium-plated PEEK feature CTL Amedica’s proprietary TiCro™ surface technology, which is designed to significantly increase contact surface area and enhance bone interlocking properties.
“We’re excited to work with our Taiwan distributor partner to make this important spinal technology readily available throughout Taiwan,” said Daniel Chon, CEO of CTL Amedica. “We hope to have it commercially available throughout Taiwan in the coming months and eventually in markets throughout Asia.”
Source: CTL Amedica
The Taiwan Food and Drug Administration (TFDA) granted a license to CTL Amedica for the MATISSE™ Anterior Cervical Interbody Fusion (ACIF) Cage. The official license, DHA 05603556709, enables CTL Amedica to market the MATISSE ACIF Cage in Taiwan.
The MATISSE ACIF Cage is engineered to accommodate a wide range of patient anatomies and surgeon...
The Taiwan Food and Drug Administration (TFDA) granted a license to CTL Amedica for the MATISSE™ Anterior Cervical Interbody Fusion (ACIF) Cage. The official license, DHA 05603556709, enables CTL Amedica to market the MATISSE ACIF Cage in Taiwan.
The MATISSE ACIF Cage is engineered to accommodate a wide range of patient anatomies and surgeon preferences. It features streamlined instrumentation and a tapered leading edge for easy insertion, as well as a large graft area to further promote bone fusion.
The system is available in numerous footprints, heights, lordotic angles and materials, including titanium, PEEK and titanium-plated PEEK. Both titanium and titanium-plated PEEK feature CTL Amedica’s proprietary TiCro™ surface technology, which is designed to significantly increase contact surface area and enhance bone interlocking properties.
“We’re excited to work with our Taiwan distributor partner to make this important spinal technology readily available throughout Taiwan,” said Daniel Chon, CEO of CTL Amedica. “We hope to have it commercially available throughout Taiwan in the coming months and eventually in markets throughout Asia.”
Source: CTL Amedica

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







