
 Copy to clipboard
Copy to clipboard 
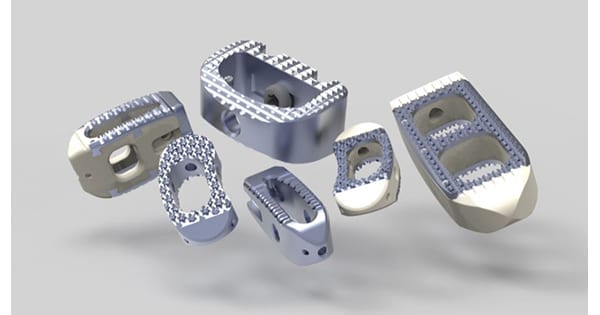
CTL Amedica received FDA 510(k) clearance to market its MONDRIAN™ Lumbar Interbody Fusion (LIF) Cage, featuring TiCro™ surface technology.
TiCro surface architecture is a proprietary approach to machined titanium technologies. The surface geometry is intended to enhance interlocking properties and increase bone ingrowth surface area by 200% over predicates.
In the MONDRIAN LIF line, the titanium bullet TLIF and PLIF with TiCro are slated for limited launch in 4Q20, with other formats to follow.
MONDRIAN LIF features a large graft window, radiographic markers, a self-distracting bullet tip and a bi-convex profile and lateral aperture. The system is designed for use with supplemental fixation.
Daniel Chon, CTL Amedica Corporation President and CEO, said, “With multiple material offerings, 15 different cage configurations, 34 footprints, 7 lordotic options and 7,160 part combinations, the sheer breadth of this clearance is a tremendous accomplishment and a testament to our team’s dedication to ‘moving the needle’ in product innovation and design.”
CTL Amedica received FDA 510(k) clearance to market its MONDRIAN™ Lumbar Interbody Fusion (LIF) Cage, featuring TiCro™ surface technology.
TiCro surface architecture is a proprietary approach to machined titanium technologies. The surface geometry is intended to enhance interlocking properties and increase bone ingrowth surface area by 200%...
CTL Amedica received FDA 510(k) clearance to market its MONDRIAN™ Lumbar Interbody Fusion (LIF) Cage, featuring TiCro™ surface technology.
TiCro surface architecture is a proprietary approach to machined titanium technologies. The surface geometry is intended to enhance interlocking properties and increase bone ingrowth surface area by 200% over predicates.
In the MONDRIAN LIF line, the titanium bullet TLIF and PLIF with TiCro are slated for limited launch in 4Q20, with other formats to follow.
MONDRIAN LIF features a large graft window, radiographic markers, a self-distracting bullet tip and a bi-convex profile and lateral aperture. The system is designed for use with supplemental fixation.
Daniel Chon, CTL Amedica Corporation President and CEO, said, “With multiple material offerings, 15 different cage configurations, 34 footprints, 7 lordotic options and 7,160 part combinations, the sheer breadth of this clearance is a tremendous accomplishment and a testament to our team’s dedication to ‘moving the needle’ in product innovation and design.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







