Copy to clipboard
Copy to clipboard 
The Guatemala Ministry of Public Health and Social Assistance has certified CTL Amedica’s registration for two product lines: VALEO™ II TL Silicon Nitride Interbody Fusion devices and the RAPHAEL™ Pedicle Fixation Platform. This announcement marks CTL Amedica’s first product registration certification in Central America, and the company says there is more to come.
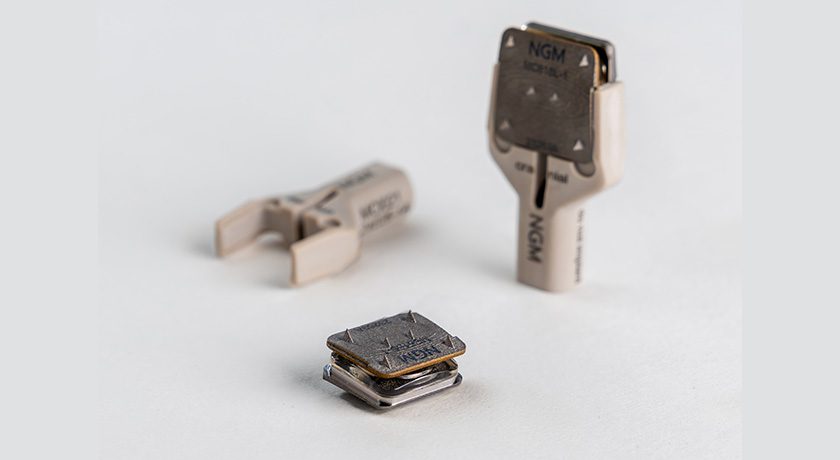
VALEO II TL (shown above) and RAPHEL are slated to launch in Guatemala immediately.
VALEO II TL is a transforaminal lumbar interbody fusion (TLIF) device. VALEO II TL interbody cages are manufactured from silicon nitride, which is designed to demonstrate unique bacteriostatic properties, provide superior imaging across all modalities and promote an enhanced osteogenic response. CTL Amedica is the world’s exclusive provider of silicon nitride spine products.
RAPHAEL is an open pedicle fixation platform comprising a range of polyaxial screws; dual lead and cortical threading; various rods, connectors and hooks and locking set screws, together providing rigid fixation in posterior lumbar fusion.
“We’re grateful to the Guatemala Ministry of Public Health and Social Assistance for the opportunity to make this important technology available in Guatemala. At CTL Amedica, we design, develop and manufacture innovative medical devices that are efficient and effective for surgeons, all while keeping patients at the forefront of our minds. Our vision is to continue expanding our global footprint, so our novel and streamlined technologies are more accessible internationally,” said Daniel Chon, CTL Amedica President and CEO.
Source: CTL Amedica
The Guatemala Ministry of Public Health and Social Assistance has certified CTL Amedica's registration for two product lines: VALEO™ II TL Silicon Nitride Interbody Fusion devices and the RAPHAEL™ Pedicle Fixation Platform. This announcement marks CTL Amedica’s first product registration certification in Central America, and the company says...
The Guatemala Ministry of Public Health and Social Assistance has certified CTL Amedica’s registration for two product lines: VALEO™ II TL Silicon Nitride Interbody Fusion devices and the RAPHAEL™ Pedicle Fixation Platform. This announcement marks CTL Amedica’s first product registration certification in Central America, and the company says there is more to come.
VALEO II TL (shown above) and RAPHEL are slated to launch in Guatemala immediately.
VALEO II TL is a transforaminal lumbar interbody fusion (TLIF) device. VALEO II TL interbody cages are manufactured from silicon nitride, which is designed to demonstrate unique bacteriostatic properties, provide superior imaging across all modalities and promote an enhanced osteogenic response. CTL Amedica is the world’s exclusive provider of silicon nitride spine products.
RAPHAEL is an open pedicle fixation platform comprising a range of polyaxial screws; dual lead and cortical threading; various rods, connectors and hooks and locking set screws, together providing rigid fixation in posterior lumbar fusion.
“We’re grateful to the Guatemala Ministry of Public Health and Social Assistance for the opportunity to make this important technology available in Guatemala. At CTL Amedica, we design, develop and manufacture innovative medical devices that are efficient and effective for surgeons, all while keeping patients at the forefront of our minds. Our vision is to continue expanding our global footprint, so our novel and streamlined technologies are more accessible internationally,” said Daniel Chon, CTL Amedica President and CEO.
Source: CTL Amedica

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.