
 Copy to clipboard
Copy to clipboard 
CrossRoads Extremity Systems received FDA clearance to market the DynaFORCE® Active Stabilization™ MPJ Implant, reportedly the only system on the market that combines an anatomic plate with active compression from a nitinol clip, delivered sterile-packed to treat metatarsophalangeal joints.
CrossRoads launched the first DynaFORCE hybrid fixation system in 2017, followed by the Active Stabilization™ Vero Medial Column Implant in 1Q18.
Source: CrossRoads Extremity Systems
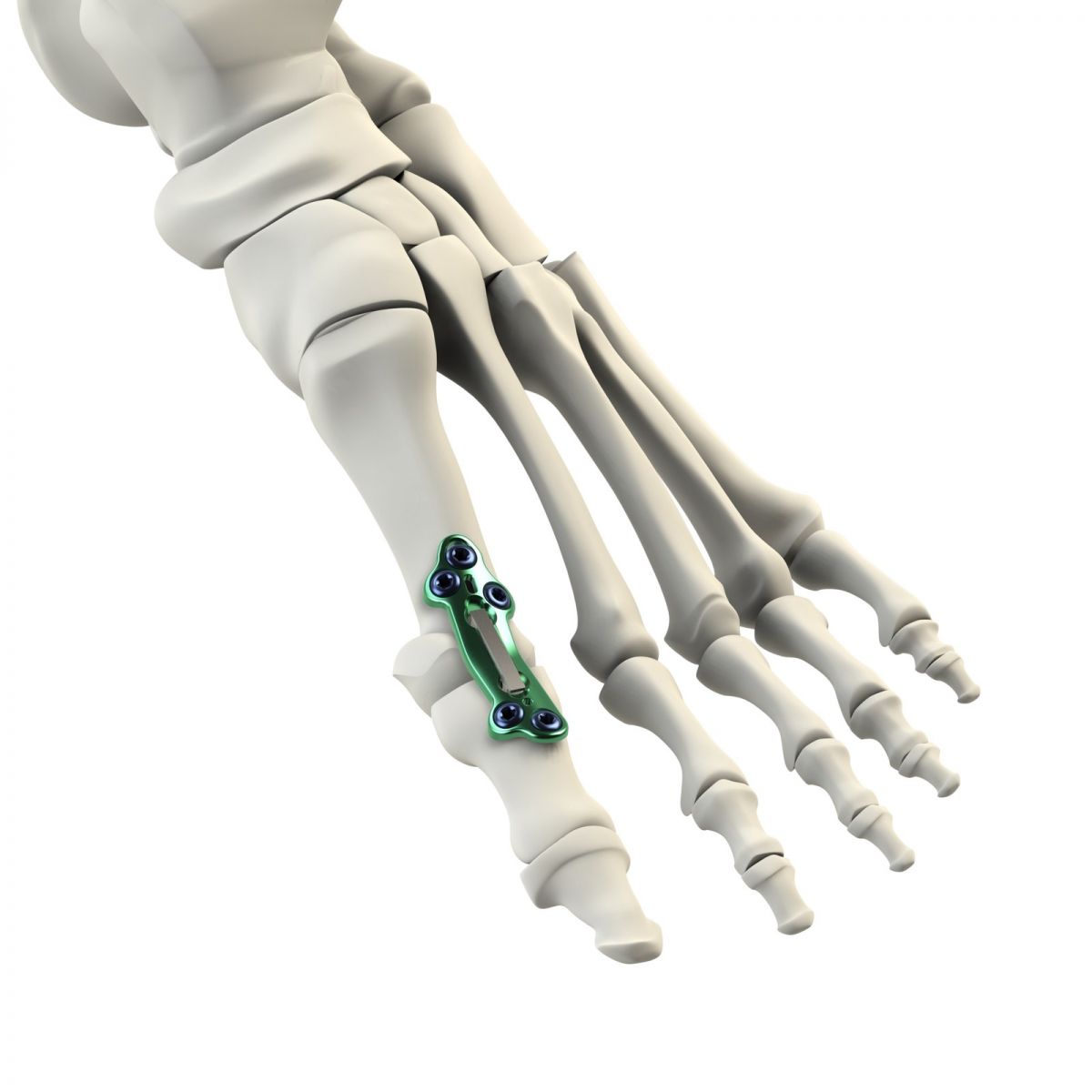
Image courtesy of CrossRoads Extremity Systems
CrossRoads Extremity Systems received FDA clearance to market the DynaFORCE® Active Stabilization™ MPJ Implant, reportedly the only system on the market that combines an anatomic plate with active compression from a nitinol clip, delivered sterile-packed to treat metatarsophalangeal joints.
CrossRoads launched the first DynaFORCE hybrid...
CrossRoads Extremity Systems received FDA clearance to market the DynaFORCE® Active Stabilization™ MPJ Implant, reportedly the only system on the market that combines an anatomic plate with active compression from a nitinol clip, delivered sterile-packed to treat metatarsophalangeal joints.
CrossRoads launched the first DynaFORCE hybrid fixation system in 2017, followed by the Active Stabilization™ Vero Medial Column Implant in 1Q18.
Source: CrossRoads Extremity Systems

Image courtesy of CrossRoads Extremity Systems

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.






