
 Copy to clipboard
Copy to clipboard 
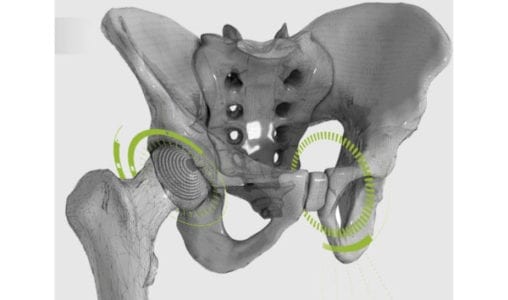
Corin received FDA 510(k) clearance for OPSInsight™, cloud-based software that allows surgeons to access and adjust customized pre-operative plans for total hip arthroplasty. This is the latest evolution of the company’s Optimized Positioning System (OPS™), which uses pre-operative functional imaging to plan optimal implant alignment while considering individual patient anatomy, spinopelvic mobility and functional biomechanics.
OPSInsight, part of the CorinConnect™ ecosystem, supports access to additional information like advanced spinopelvic assessment, impingement analysis and radiodensity mapping.
Personalized technologies deployed within CorinConnect generate actionable insights on a customizable dashboard before, during and after the procedure.
Corin’s Chief Innovation Officer, Jim Pierrepont, Ph.D., said, “Our belief is that OPSInsight is the new gold standard in THA planning. The possible depth of analysis is unparalleled, and key insights are presented in an intuitive and user-friendly interface to our surgeons. We believe this level of planning is a necessity to improve outcomes in THA.”
Corin received FDA 510(k) clearance for OPSInsight™, cloud-based software that allows surgeons to access and adjust customized pre-operative plans for total hip arthroplasty. This is the latest evolution of the company's Optimized Positioning System (OPS™), which uses pre-operative functional imaging to plan optimal implant alignment while...
Corin received FDA 510(k) clearance for OPSInsight™, cloud-based software that allows surgeons to access and adjust customized pre-operative plans for total hip arthroplasty. This is the latest evolution of the company’s Optimized Positioning System (OPS™), which uses pre-operative functional imaging to plan optimal implant alignment while considering individual patient anatomy, spinopelvic mobility and functional biomechanics.
OPSInsight, part of the CorinConnect™ ecosystem, supports access to additional information like advanced spinopelvic assessment, impingement analysis and radiodensity mapping.
Personalized technologies deployed within CorinConnect generate actionable insights on a customizable dashboard before, during and after the procedure.
Corin’s Chief Innovation Officer, Jim Pierrepont, Ph.D., said, “Our belief is that OPSInsight is the new gold standard in THA planning. The possible depth of analysis is unparalleled, and key insights are presented in an intuitive and user-friendly interface to our surgeons. We believe this level of planning is a necessity to improve outcomes in THA.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







