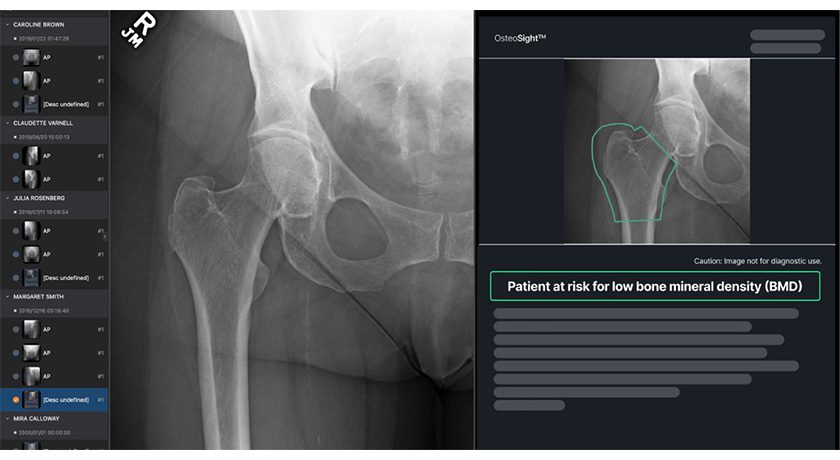
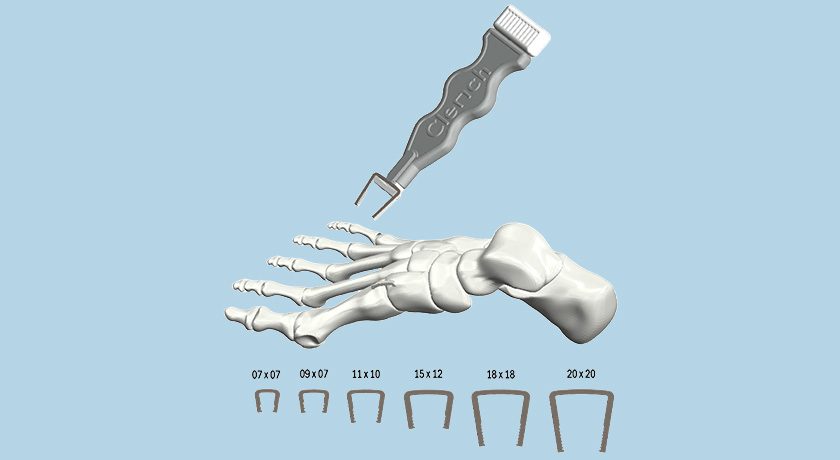
Copy to clipboard
Copy to clipboard 
Corin commenced U.S. launch of the Humelock™ Reversed Shoulder, designed to treat massive rotator cuff tears and severe arthritis.
The Humelock Reversed Shoulder received FDA 510(k) clearance in January 2017, and was previously available in the EU and other ex-U.S. markets.
Corin holds a U.S. distribution agreement with FX Solutions encompassing the Humelock II Cementless, Cemented Anatomical and Reversible Fracture Shoulder Systems, plus additional products pending FDA clearance. The agreement includes distribution rights in Germany and the U.K.
Sources: Corin Group; ORTHOWORLD Inc.
Corin commenced U.S. launch of the Humelock™ Reversed Shoulder, designed to treat massive rotator cuff tears and severe arthritis.
The Humelock Reversed Shoulder received FDA 510(k) clearance in January 2017, and was previously available in the EU and other ex-U.S. markets.
Corin holds a U.S. distribution agreement with FX Solutions...
Corin commenced U.S. launch of the Humelock™ Reversed Shoulder, designed to treat massive rotator cuff tears and severe arthritis.
The Humelock Reversed Shoulder received FDA 510(k) clearance in January 2017, and was previously available in the EU and other ex-U.S. markets.
Corin holds a U.S. distribution agreement with FX Solutions encompassing the Humelock II Cementless, Cemented Anatomical and Reversible Fracture Shoulder Systems, plus additional products pending FDA clearance. The agreement includes distribution rights in Germany and the U.K.
Sources: Corin Group; ORTHOWORLD Inc.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.