
 Copy to clipboard
Copy to clipboard 
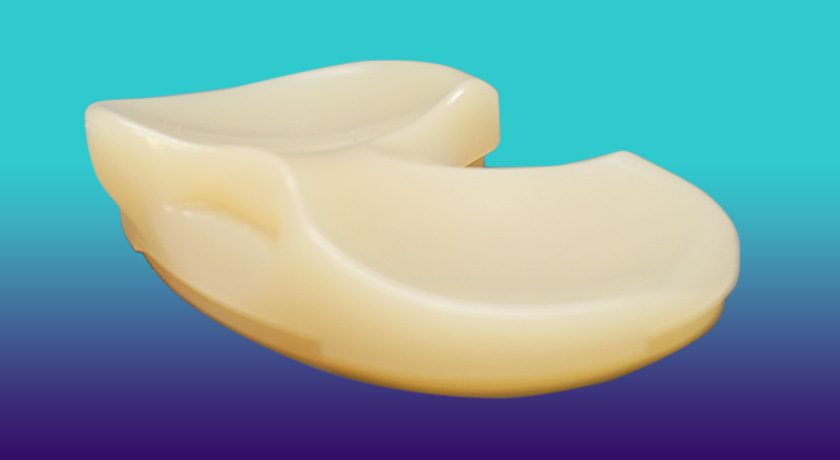
Corin was granted FDA 510(k) clearance to market the Unity Knee Medial Constrained (MC) Tibial Insert, which offers the option to independently stabilize the medial compartment during a standard or robotic-assisted total knee replacement.
The Unity Knee MC Tibial Insert was designed to help restore native knee kinematics by stabilizing the medial condyle while allowing lateral translation and is suitable for use with or without the posterior cruciate ligament. Used in over 75,000 surgical cases since its 2012 launch and demonstrating some of the best performance across the UK & Australian implant registries, the Unity Knee system continues to grow.
The insert is manufactured with ECiMa bearing technology, a vitamin-E enriched, highly cross-linked polyethylene that provides low wear characteristics, oxidative stability and enhanced mechanical performance. As part of the Unity Knee system, it is compatible with ApolloKnee, Corin’s next-generation robotic-assisted surgical platform.
ApolloKnee provides an objective, dual compartment, sensing and tensing balance assessment prior to bone resections, allowing surgeons to optimize knee function in real-time. The combination of advanced implant design and unique robotic-assisted balancing aims to improve patient outcomes and satisfaction.
The Unity Knee MC Tibial Insert is exclusively available in the United States through a limited market release.
Jim Pierrepont, Global Franchise Lead at Corin, said, “We’re also anticipating further clearances in the coming months which will strengthen our implant, Apollo and CorinConnect digital portfolios.”
“Studies have demonstrated that a balanced joint has a greater impact on total knee replacement outcomes than component alignment alone. Combining Unity Knee MC with the robotic soft tissue balancing capabilities of ApolloKnee, a new level of natural joint kinematics and stability can be achieved throughout the range of motion. We believe this will provide patients with a more natural feeling knee.” said Christopher Plaskos, PhD, VP, Global Clinical Innovation at Corin.
Source: Corin Group
Corin was granted FDA 510(k) clearance to market the Unity Knee Medial Constrained (MC) Tibial Insert, which offers the option to independently stabilize the medial compartment during a standard or robotic-assisted total knee replacement.
The Unity Knee MC Tibial Insert was designed to help restore native knee kinematics by stabilizing the...
Corin was granted FDA 510(k) clearance to market the Unity Knee Medial Constrained (MC) Tibial Insert, which offers the option to independently stabilize the medial compartment during a standard or robotic-assisted total knee replacement.
The Unity Knee MC Tibial Insert was designed to help restore native knee kinematics by stabilizing the medial condyle while allowing lateral translation and is suitable for use with or without the posterior cruciate ligament. Used in over 75,000 surgical cases since its 2012 launch and demonstrating some of the best performance across the UK & Australian implant registries, the Unity Knee system continues to grow.
The insert is manufactured with ECiMa bearing technology, a vitamin-E enriched, highly cross-linked polyethylene that provides low wear characteristics, oxidative stability and enhanced mechanical performance. As part of the Unity Knee system, it is compatible with ApolloKnee, Corin’s next-generation robotic-assisted surgical platform.
ApolloKnee provides an objective, dual compartment, sensing and tensing balance assessment prior to bone resections, allowing surgeons to optimize knee function in real-time. The combination of advanced implant design and unique robotic-assisted balancing aims to improve patient outcomes and satisfaction.
The Unity Knee MC Tibial Insert is exclusively available in the United States through a limited market release.
Jim Pierrepont, Global Franchise Lead at Corin, said, “We’re also anticipating further clearances in the coming months which will strengthen our implant, Apollo and CorinConnect digital portfolios.”
“Studies have demonstrated that a balanced joint has a greater impact on total knee replacement outcomes than component alignment alone. Combining Unity Knee MC with the robotic soft tissue balancing capabilities of ApolloKnee, a new level of natural joint kinematics and stability can be achieved throughout the range of motion. We believe this will provide patients with a more natural feeling knee.” said Christopher Plaskos, PhD, VP, Global Clinical Innovation at Corin.
Source: Corin Group

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







