
 Copy to clipboard
Copy to clipboard 
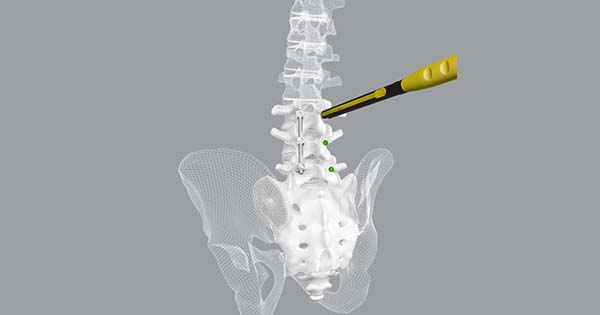
CoreLink announced FDA 510(k) clearance and U.S. launch of CoreLink CentraFix™ Midline Fixation.
CentraFix is a posterior thoracolumbar pedicle screw system designed for less invasive spinal fixation often used with a medial-to-lateral approach, known as cortical bone trajectory. This technique maximizes contact of the pedicle screw with cortical bone and is intended to reduce incision size, limit muscular and vascular injury, and improve initial fixation. CentraFix features modular cobalt chrome tulip heads and titanium alloy screw shanks in various lengths and diameters, designed specifically to allow screw placement in denser cortical bone.
Self-drilling, self-tapping cortical screw threading provides easy screw starting in the intended trajectory and allow for 360° motion with a 60° cone of angulation. The system includes 4.75mm and 5.5mm rod options, set screws designed to minimize tulip splay, and a robust offering of ergonomic and intuitive instrumentation to facilitate fast and efficient surgery.
Source: CoreLink
CoreLink announced FDA 510(k) clearance and U.S. launch of CoreLink CentraFix™ Midline Fixation.
CentraFix is a posterior thoracolumbar pedicle screw system designed for less invasive spinal fixation often used with a medial-to-lateral approach, known as cortical bone trajectory. This technique maximizes contact of the pedicle screw with...
CoreLink announced FDA 510(k) clearance and U.S. launch of CoreLink CentraFix™ Midline Fixation.
CentraFix is a posterior thoracolumbar pedicle screw system designed for less invasive spinal fixation often used with a medial-to-lateral approach, known as cortical bone trajectory. This technique maximizes contact of the pedicle screw with cortical bone and is intended to reduce incision size, limit muscular and vascular injury, and improve initial fixation. CentraFix features modular cobalt chrome tulip heads and titanium alloy screw shanks in various lengths and diameters, designed specifically to allow screw placement in denser cortical bone.
Self-drilling, self-tapping cortical screw threading provides easy screw starting in the intended trajectory and allow for 360° motion with a 60° cone of angulation. The system includes 4.75mm and 5.5mm rod options, set screws designed to minimize tulip splay, and a robust offering of ergonomic and intuitive instrumentation to facilitate fast and efficient surgery.
Source: CoreLink

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







