
 Copy to clipboard
Copy to clipboard 
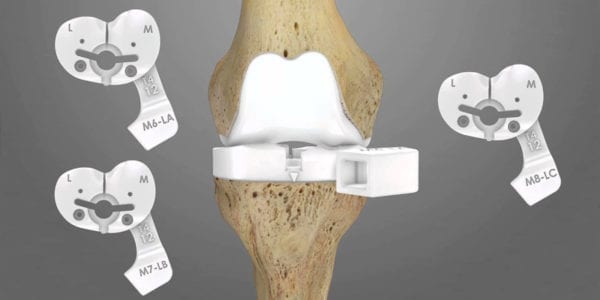
Conformis reached the milestone of 100,000 knee joint replacement implants manufactured and sold in the U.S.
The company’s knee systems are complemented by a new 3D-designed hip, which gained FDA 510(k) clearance in 4Q19. Like its knee technology, the hip employs proprietary imaging and design software to create a patient-specific pre-operative surgical plan for use with iJig instruments and patient-matched implants. The instruments work with anatomic landmarks to help place and orient the femoral stem and acetabular cup. A proprietary single-use reamer enables patient-matched reaming.
Over 20 peer-reviewed published clinical studies support the strong performance of Conformis technology. In 2018, the company engaged PrecisionEffect and SurveyHealthcareGlobus to evaluate patient satisfaction with Conformis vs. non-Conformis knee devices. Results indicate that 87% of Conformis patients were highly likely to recommend their implant to others, vs. 70% of non-Conformis patients surveyed.
“We are very pleased to announce this tremendous milestone for personalized joint replacement implant treatments,” said Mark Augusti, President and Chief Executive Officer. “We are strong advocates for the utilization of patient-specific implants in total knee replacement because the size, shape and curvature of the knee are unique for each patient. Implants designed specifically for each patient enable efficient surgery and higher levels of patient satisfaction versus standard ‘off-the-shelf’ implants. Thank you to all of the surgeons and patients who have traveled this journey with us and helped achieve this significant milestone.”
Conformis reached the milestone of 100,000 knee joint replacement implants manufactured and sold in the U.S.
The company's knee systems are complemented by a new 3D-designed hip, which gained FDA 510(k) clearance in 4Q19. Like its knee technology, the hip employs proprietary imaging and design software to create a patient-specific...
Conformis reached the milestone of 100,000 knee joint replacement implants manufactured and sold in the U.S.
The company’s knee systems are complemented by a new 3D-designed hip, which gained FDA 510(k) clearance in 4Q19. Like its knee technology, the hip employs proprietary imaging and design software to create a patient-specific pre-operative surgical plan for use with iJig instruments and patient-matched implants. The instruments work with anatomic landmarks to help place and orient the femoral stem and acetabular cup. A proprietary single-use reamer enables patient-matched reaming.
Over 20 peer-reviewed published clinical studies support the strong performance of Conformis technology. In 2018, the company engaged PrecisionEffect and SurveyHealthcareGlobus to evaluate patient satisfaction with Conformis vs. non-Conformis knee devices. Results indicate that 87% of Conformis patients were highly likely to recommend their implant to others, vs. 70% of non-Conformis patients surveyed.
“We are very pleased to announce this tremendous milestone for personalized joint replacement implant treatments,” said Mark Augusti, President and Chief Executive Officer. “We are strong advocates for the utilization of patient-specific implants in total knee replacement because the size, shape and curvature of the knee are unique for each patient. Implants designed specifically for each patient enable efficient surgery and higher levels of patient satisfaction versus standard ‘off-the-shelf’ implants. Thank you to all of the surgeons and patients who have traveled this journey with us and helped achieve this significant milestone.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







