
 Copy to clipboard
Copy to clipboard 
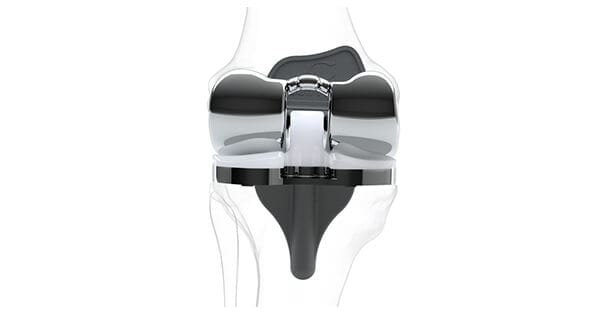
Conformis received regulatory clearance of its patient-specific iTotal® PS total knee replacement system by the Therapeutic Goods Administration in Australia.
iTotal PS, which launched in the U.S. in 2016, is designed as an alternative to off-the-shelf knee replacements that are manufactured in limited sizes and shapes. iTotal PS implants use a customized cam and spine feature to serve the function of the posterior cruciate ligament and provide optimal stability throughout the full range of motion.
“Receiving clearance for our iTotal PS product in Australia will allow us to offer a best-in-class, patient-specific solution to relieve patients’ chronic knee joint pain,” said Mark Augusti, President and CEO. “With this clearance, we have greatly expanded the number of potential patients who will be candidates for our personalized knee replacement implants, and we are thrilled to offer the benefits of our unique technology to surgeons in Australia who prefer a posterior-stabilized design to a cruciate-retaining one.”
Conformis received regulatory clearance of its patient-specific iTotal® PS total knee replacement system by the Therapeutic Goods Administration in Australia.
iTotal PS, which launched in the U.S. in 2016, is designed as an alternative to off-the-shelf knee replacements that are manufactured in limited sizes and shapes. iTotal PS implants...
Conformis received regulatory clearance of its patient-specific iTotal® PS total knee replacement system by the Therapeutic Goods Administration in Australia.
iTotal PS, which launched in the U.S. in 2016, is designed as an alternative to off-the-shelf knee replacements that are manufactured in limited sizes and shapes. iTotal PS implants use a customized cam and spine feature to serve the function of the posterior cruciate ligament and provide optimal stability throughout the full range of motion.
“Receiving clearance for our iTotal PS product in Australia will allow us to offer a best-in-class, patient-specific solution to relieve patients’ chronic knee joint pain,” said Mark Augusti, President and CEO. “With this clearance, we have greatly expanded the number of potential patients who will be candidates for our personalized knee replacement implants, and we are thrilled to offer the benefits of our unique technology to surgeons in Australia who prefer a posterior-stabilized design to a cruciate-retaining one.”

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







