Copy to clipboard
Copy to clipboard 
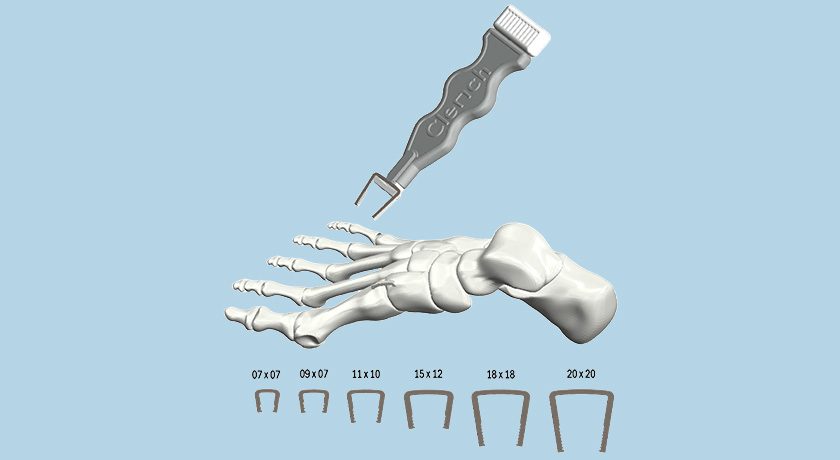
Conformis entered into an agreement with Paragon 28, granting the company a non-exclusive license under a subset of Conformis’ U.S. patents for the use of patient-specific instruments with off-the-shelf implants in Paragon 28’s APEX 3D™ Total Ankle.
The APEX system gained FDA 510(k) clearance in 2020.
“We are pleased to reach this agreement with Paragon 28, enabling it to incorporate our patient-specific instrument technology in its anticipated total ankle replacement offering,” said Mark Augusti, President and Chief Executive Officer. “We believe that this latest license agreement further demonstrates the broad applicability and clinical appeal of Conformis’ technology.”
Conformis entered into an agreement with Paragon 28, granting the company a non-exclusive license under a subset of Conformis’ U.S. patents for the use of patient-specific instruments with off-the-shelf implants in Paragon 28’s APEX 3D™ Total Ankle.
The APEX system gained FDA 510(k) clearance in 2020.
“We are pleased to reach this...
Conformis entered into an agreement with Paragon 28, granting the company a non-exclusive license under a subset of Conformis’ U.S. patents for the use of patient-specific instruments with off-the-shelf implants in Paragon 28’s APEX 3D™ Total Ankle.
The APEX system gained FDA 510(k) clearance in 2020.
“We are pleased to reach this agreement with Paragon 28, enabling it to incorporate our patient-specific instrument technology in its anticipated total ankle replacement offering,” said Mark Augusti, President and Chief Executive Officer. “We believe that this latest license agreement further demonstrates the broad applicability and clinical appeal of Conformis’ technology.”

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.