
 Copy to clipboard
Copy to clipboard 
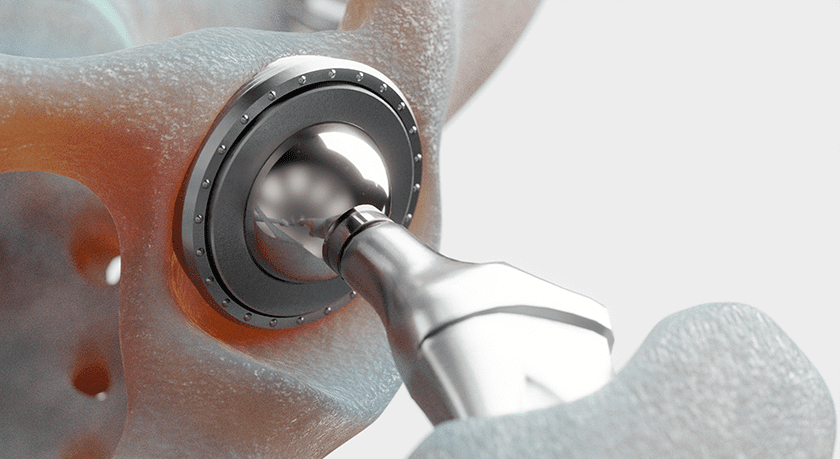
Conformis received FDA 510(k) clearance to market the Actera™ Hip System.
Actera adds a tri-taper femoral stem design to the expanding Conformis hip portfolio. This is intended to facilitate a minimally invasive approach similar to direct anterior, with easier access to the femur and consequently less injury to muscles and fewer potential interactions with nerves.
Conformis will introduce the Actera hip under a limited market release in select U.S. markets, which is expected to commence in the coming months.
Chronology of the Conformis Hip Portfolio:
- Conformis Actera Hip System: Utilizes an advanced tri-taper femur stem design that facilitates direct anterior approach total hip arthroplasties, and provides an additional stem option to orthopedic surgeons.
- Conformis Cordera™ Hip System: Received FDA 510(k) marketing clearance in 2020. It is a cementless primary total hip replacement composed of femoral (thigh) and acetabular (socket of the hip bone) components. The system can be used with or without a pre-operative CT scan that is used to design Conformis iJigs® (patient-specific instruments) and a personalized surgical plan.
- Conformis Hip System: Received FDA 510(k) marketing clearance in 2019. The Conformis Hip System is reportedly the only primary total hip replacement system on the market designed with 3D imaging technology to provide a stem and acetabular cup size that matches each patient’s specific anatomy.
Source: Conformis, Inc.
Conformis received FDA 510(k) clearance to market the Actera™ Hip System.
Actera adds a tri-taper femoral stem design to the expanding Conformis hip portfolio. This is intended to facilitate a minimally invasive approach similar to direct anterior, with easier access to the femur and consequently less injury to muscles and fewer potential...
Conformis received FDA 510(k) clearance to market the Actera™ Hip System.
Actera adds a tri-taper femoral stem design to the expanding Conformis hip portfolio. This is intended to facilitate a minimally invasive approach similar to direct anterior, with easier access to the femur and consequently less injury to muscles and fewer potential interactions with nerves.
Conformis will introduce the Actera hip under a limited market release in select U.S. markets, which is expected to commence in the coming months.
Chronology of the Conformis Hip Portfolio:
- Conformis Actera Hip System: Utilizes an advanced tri-taper femur stem design that facilitates direct anterior approach total hip arthroplasties, and provides an additional stem option to orthopedic surgeons.
- Conformis Cordera™ Hip System: Received FDA 510(k) marketing clearance in 2020. It is a cementless primary total hip replacement composed of femoral (thigh) and acetabular (socket of the hip bone) components. The system can be used with or without a pre-operative CT scan that is used to design Conformis iJigs® (patient-specific instruments) and a personalized surgical plan.
- Conformis Hip System: Received FDA 510(k) marketing clearance in 2019. The Conformis Hip System is reportedly the only primary total hip replacement system on the market designed with 3D imaging technology to provide a stem and acetabular cup size that matches each patient’s specific anatomy.
Source: Conformis, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







