
 Copy to clipboard
Copy to clipboard 
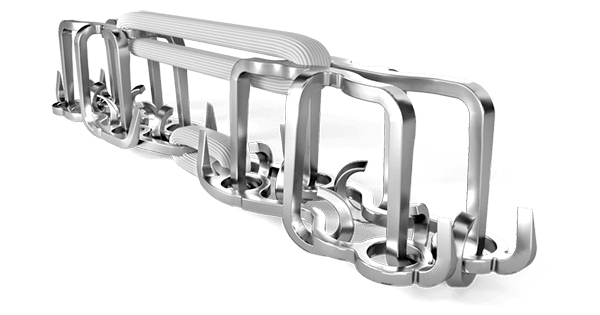
CoNextions was granted FDA 510(k) clearance to market the CoNextions TR® Tendon Repair System. Expected to launch in May 2022, the novel system addresses traumatic tendon lacerations in the hand, wrist and forearm.
Studies have demonstrated that CoNextions TR supports a strong, fast and smooth repair of tendon lacerations. A prospective randomized clinical trial also showed a lower rate of treatment failure and rupture compared to conventional suture repairs.
“We are pleased with the outcome of FDA’s review of our 510(k) submission supported by a ninety-patient clinical trial, and are excited about the upcoming introduction and commercial launch of the product,” said Jeffrey Barnes, President and CEO of CoNextions.
Source: CoNextions Inc.
CoNextions was granted FDA 510(k) clearance to market the CoNextions TR® Tendon Repair System. Expected to launch in May 2022, the novel system addresses traumatic tendon lacerations in the hand, wrist and forearm.
Studies have demonstrated that CoNextions TR supports a strong, fast and smooth repair of tendon lacerations. A prospective...
CoNextions was granted FDA 510(k) clearance to market the CoNextions TR® Tendon Repair System. Expected to launch in May 2022, the novel system addresses traumatic tendon lacerations in the hand, wrist and forearm.
Studies have demonstrated that CoNextions TR supports a strong, fast and smooth repair of tendon lacerations. A prospective randomized clinical trial also showed a lower rate of treatment failure and rupture compared to conventional suture repairs.
“We are pleased with the outcome of FDA’s review of our 510(k) submission supported by a ninety-patient clinical trial, and are excited about the upcoming introduction and commercial launch of the product,” said Jeffrey Barnes, President and CEO of CoNextions.
Source: CoNextions Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







