Copy to clipboard
Copy to clipboard 
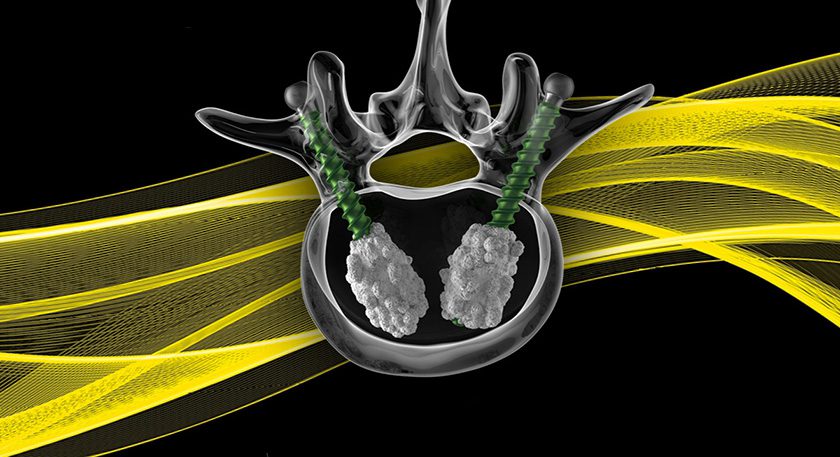
ChoiceSpine received FDA 510(k) clearance to market the TIGER SHARK™ C interbody device with BioBond™ technology and the BOOMERANG™ Anterior Cervical Plate (ACP), for use together or separately in cervical spinal fusion.
BOOMERANG instrumentation is designed to allow simultaneous insertion of the BOOMERANG plate with cervical interbodies, such as TIGER SHARK C or STEALTH™, mimicking a stand-alone ACDF concept and reducing O.R. time. The BOOMERANG ACP features a two-screw configuration and single-step screw blocking mechanism to optimize insertion and fixation.
TIGER SHARK C is a 3D-manufactured titanium alloy interbody created with BioBond organic, porous interbody architecture designed for continuous porosity with an osteoconductive, hydrophilic surface.
Source: ChoiceSpine
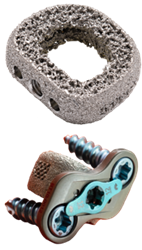
ChoiceSpine received FDA 510(k) clearance to market the TIGER SHARK™ C interbody device with BioBond™ technology and the BOOMERANG™ Anterior Cervical Plate (ACP), for use together or separately in cervical spinal fusion.
BOOMERANG instrumentation is designed to allow simultaneous insertion of the BOOMERANG plate with cervical interbodies, such...
ChoiceSpine received FDA 510(k) clearance to market the TIGER SHARK™ C interbody device with BioBond™ technology and the BOOMERANG™ Anterior Cervical Plate (ACP), for use together or separately in cervical spinal fusion.
BOOMERANG instrumentation is designed to allow simultaneous insertion of the BOOMERANG plate with cervical interbodies, such as TIGER SHARK C or STEALTH™, mimicking a stand-alone ACDF concept and reducing O.R. time. The BOOMERANG ACP features a two-screw configuration and single-step screw blocking mechanism to optimize insertion and fixation.
TIGER SHARK C is a 3D-manufactured titanium alloy interbody created with BioBond organic, porous interbody architecture designed for continuous porosity with an osteoconductive, hydrophilic surface.

Source: ChoiceSpine

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.