
 Copy to clipboard
Copy to clipboard 
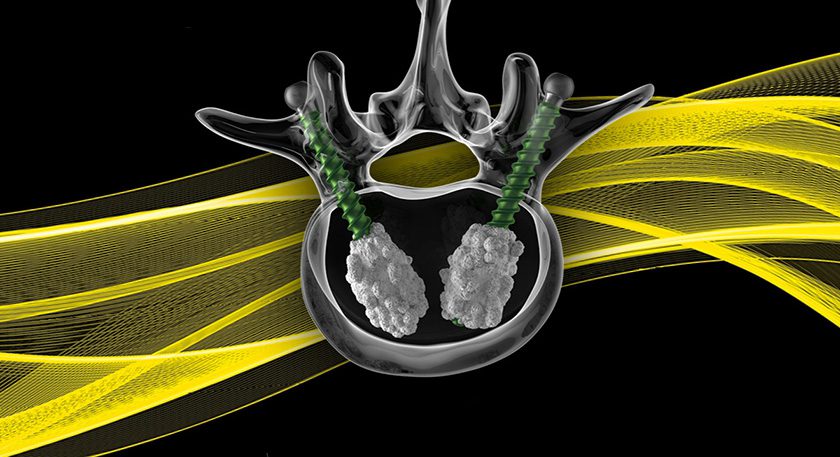
CGBio’s NOVOSIS PUTTY was granted Breakthrough Device Designation by FDA. This material incorporates recombinant human bone morphogenetic protein 2 (rhBMP-2). NOVOSIS PUTTY’s designation marks the first case for an implantable device in Korea.
NOVOSIS PUTTY, a second-generation product, features a ceramic-based synthetic scaffold with superior moldability and osteoconductive properties, and incorporates advanced sustained-release formulation technology (SLOREL) from its predecessor, NOVOSIS Ortho.
The rhBMP-2 ingredient plays a critical role in bone regeneration, transforming stem cells into bone cells in cases of bone defects. Hydroxyapatite ceramic material acts as a scaffold for rhBMP-2, allowing for controlled release of rhBMP-2, reducing the risk of unwanted bone growth and surrounding soft tissue swelling. It enables high-density bone formation with lower rhBMP-2 doses. Its synthetic polymer hydrogel component, Poloxamer 407 hydrogel, also supports customizable shaping.
CGBio has completed preclinical studies for U.S. confirmatory clinical trials of NOVOSIS PUTTY and is conducting clinical trials to determine the optimal dosage, with plans to apply for U.S. confirmatory clinical trials in the first half of 2024.
Hyun Seung Yu, CEO of CGBio, commented, “The breakthrough device designation for NOVOSIS PUTTY is further proof of CGBio’s technological prowess, innovation, and potential. With the designation’s emphasis on quickly bringing beneficial products to market, we are committed to helping more patients overcome diseases and return to healthy lives through NOVOSIS PUTTY.”
Source: CGBio
CGBio's NOVOSIS PUTTY was granted Breakthrough Device Designation by FDA. This material incorporates recombinant human bone morphogenetic protein 2 (rhBMP-2). NOVOSIS PUTTY’s designation marks the first case for an implantable device in Korea.
NOVOSIS PUTTY, a second-generation product, features a ceramic-based synthetic scaffold with superior...
CGBio’s NOVOSIS PUTTY was granted Breakthrough Device Designation by FDA. This material incorporates recombinant human bone morphogenetic protein 2 (rhBMP-2). NOVOSIS PUTTY’s designation marks the first case for an implantable device in Korea.
NOVOSIS PUTTY, a second-generation product, features a ceramic-based synthetic scaffold with superior moldability and osteoconductive properties, and incorporates advanced sustained-release formulation technology (SLOREL) from its predecessor, NOVOSIS Ortho.
The rhBMP-2 ingredient plays a critical role in bone regeneration, transforming stem cells into bone cells in cases of bone defects. Hydroxyapatite ceramic material acts as a scaffold for rhBMP-2, allowing for controlled release of rhBMP-2, reducing the risk of unwanted bone growth and surrounding soft tissue swelling. It enables high-density bone formation with lower rhBMP-2 doses. Its synthetic polymer hydrogel component, Poloxamer 407 hydrogel, also supports customizable shaping.
CGBio has completed preclinical studies for U.S. confirmatory clinical trials of NOVOSIS PUTTY and is conducting clinical trials to determine the optimal dosage, with plans to apply for U.S. confirmatory clinical trials in the first half of 2024.
Hyun Seung Yu, CEO of CGBio, commented, “The breakthrough device designation for NOVOSIS PUTTY is further proof of CGBio’s technological prowess, innovation, and potential. With the designation’s emphasis on quickly bringing beneficial products to market, we are committed to helping more patients overcome diseases and return to healthy lives through NOVOSIS PUTTY.”
Source: CGBio

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.