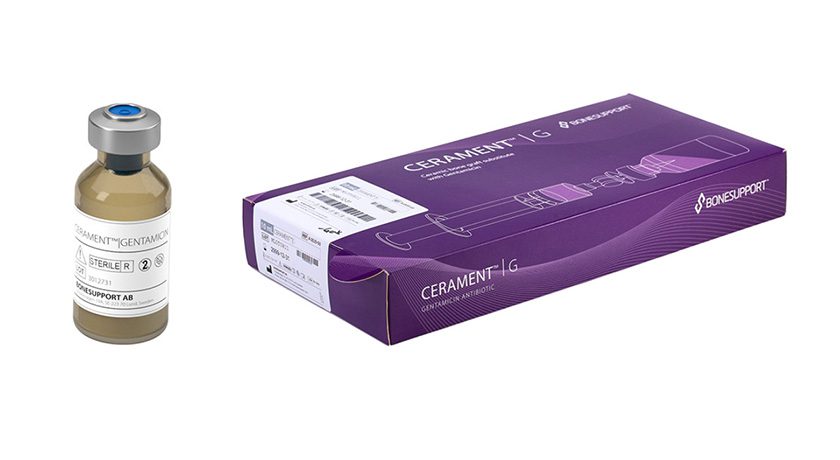






BONESUPPORT announced FDA 510(k) clearance allowing the use of CERAMENT G in open fractures.
CERAMENT G is an orthopedic medical device combination matrix consisting of a resorbable synthetic bone graft substitute and the antibiotic gentamicin, which protects against colonization of bacteria sensitive to gentamicin.
The clearance more than doubles the U.S. addressable market for CERAMENT G, which is reported to be the first and only combination product cleared in the USA for this indication.
“Open fractures are one of the most common causes of a patient developing a bone infection, and we are very excited about this expanded indication. It means that American surgeons will have a new powerful tool to treat patients with skeletal injuries while simultaneously protecting the site from infection by local elution of antibiotics,” said Emil Billbäck, CEO of BONESUPPORT.
Source: BONESUPPORT
BONESUPPORT announced FDA 510(k) clearance allowing the use of CERAMENT G in open fractures.
CERAMENT G is an orthopedic medical device combination matrix consisting of a resorbable synthetic bone graft substitute and the antibiotic gentamicin, which protects against colonization of bacteria sensitive to gentamicin.
The clearance more than...
BONESUPPORT announced FDA 510(k) clearance allowing the use of CERAMENT G in open fractures.
CERAMENT G is an orthopedic medical device combination matrix consisting of a resorbable synthetic bone graft substitute and the antibiotic gentamicin, which protects against colonization of bacteria sensitive to gentamicin.
The clearance more than doubles the U.S. addressable market for CERAMENT G, which is reported to be the first and only combination product cleared in the USA for this indication.
“Open fractures are one of the most common causes of a patient developing a bone infection, and we are very excited about this expanded indication. It means that American surgeons will have a new powerful tool to treat patients with skeletal injuries while simultaneously protecting the site from infection by local elution of antibiotics,” said Emil Billbäck, CEO of BONESUPPORT.
Source: BONESUPPORT


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.