
 Copy to clipboard
Copy to clipboard 
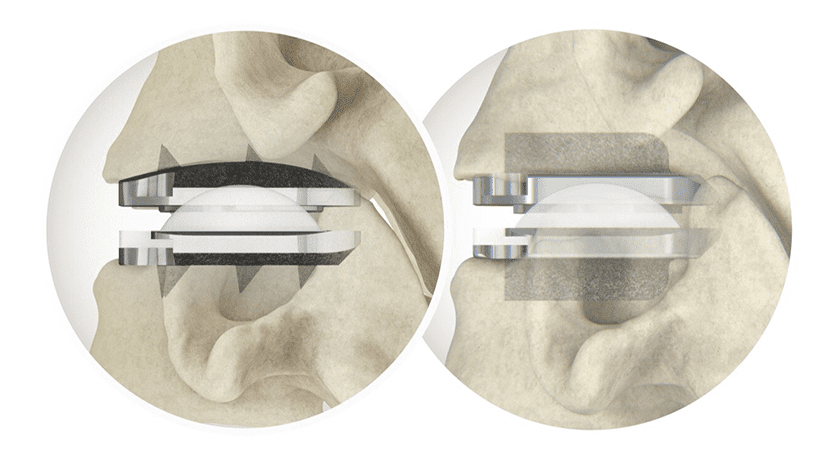
Centinel Spine announced completion of the 500th procedure in the United States with its latest FDA-approved total cervical disc replacement (TDR) system of prodisc C Vivo and prodisc C SK. The milestone comes less than two months after Centinel Spine announced the completion of 250 procedures with the system.
The prodisc C Vivo system has been in clinical use internationally since 2009 and is claimed to be one of the most frequently implanted TDR devices in the world. The device has keel-less fixation and combines an anatomically-designed superior endplate with lateral spikes to optimize fit and provide immediate fixation.
The prodisc C SK device features a flat endplate design for implant positioning that allows surgeons to address individual patient anatomy—and a low-profile central keel that provides immediate fixation and enables a streamlined keel preparation technique.
All of the prodisc cervical and lumbar devices incorporate prodisc CORE technology, the basis behind the predictable clinical outcomes of the prodisc platform after 30 years and over 225,000 implantations worldwide.
Source: Centinel Spine, LLC
Centinel Spine announced completion of the 500th procedure in the United States with its latest FDA-approved total cervical disc replacement (TDR) system of prodisc C Vivo and prodisc C SK. The milestone comes less than two months after Centinel Spine announced the completion of 250 procedures with the system.
The prodisc C Vivo system has...
Centinel Spine announced completion of the 500th procedure in the United States with its latest FDA-approved total cervical disc replacement (TDR) system of prodisc C Vivo and prodisc C SK. The milestone comes less than two months after Centinel Spine announced the completion of 250 procedures with the system.
The prodisc C Vivo system has been in clinical use internationally since 2009 and is claimed to be one of the most frequently implanted TDR devices in the world. The device has keel-less fixation and combines an anatomically-designed superior endplate with lateral spikes to optimize fit and provide immediate fixation.
The prodisc C SK device features a flat endplate design for implant positioning that allows surgeons to address individual patient anatomy—and a low-profile central keel that provides immediate fixation and enables a streamlined keel preparation technique.
All of the prodisc cervical and lumbar devices incorporate prodisc CORE technology, the basis behind the predictable clinical outcomes of the prodisc platform after 30 years and over 225,000 implantations worldwide.
Source: Centinel Spine, LLC

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







