
 Copy to clipboard
Copy to clipboard 
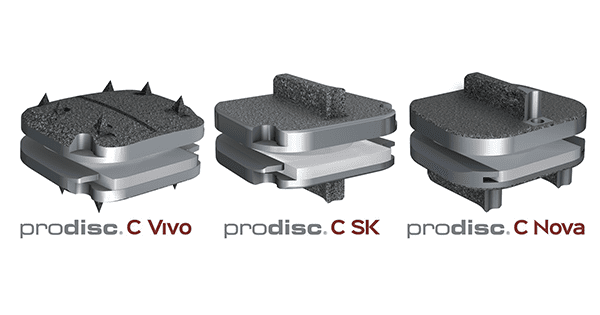
Centinel Spine announced FDA premarket application (PMA) approval for 1-level indications for three additional cervical total disc replacement (TDR) devices: prodisc C Vivo, prodisc C Nova and prodisc C SK. These join the currently available prodisc C implant, widely used throughout the U.S.
prodisc C Vivo and prodisc C Nova products have been in use outside the U.S. since 2009, and prodisc C Vivo is reportedly the most frequently implanted TDR outside of the U.S. prodisc C Vivo product has keel-less endplates including a convex, superior endplate to match more concave vertebral anatomy, while prodisc C SK and prodisc C Nova implant designs have flat endplates with low-profile keels to better match flat vertebral anatomy. All of these products incorporate prodisc CORE technology, the basis behind the predictable clinical outcomes of every prodisc device after 30 years and over 225,000 implantations, worldwide.
Centinel Spine also continues to enroll for a two-level prospective, randomized, multi-centered clinical study evaluating prodisc C Vivo and prodisc C SK.
Centinel Spine’s CEO, Steve Murray, said, “Anatomic cervical total disc options provide surgeons the benefit of selecting implants to optimally fit the disc to each patient. This is unique and represents a major advancement in spinal reconstruction. PMA approval for these three additional devices is a significant accomplishment and we look forward to bringing the new prodisc options to the market in Q4 2022.”
Source: Centinel Spine, LLC
Centinel Spine announced FDA premarket application (PMA) approval for 1-level indications for three additional cervical total disc replacement (TDR) devices: prodisc C Vivo, prodisc C Nova and prodisc C SK. These join the currently available prodisc C implant, widely used throughout the U.S.
prodisc C Vivo and prodisc C Nova products have been...
Centinel Spine announced FDA premarket application (PMA) approval for 1-level indications for three additional cervical total disc replacement (TDR) devices: prodisc C Vivo, prodisc C Nova and prodisc C SK. These join the currently available prodisc C implant, widely used throughout the U.S.
prodisc C Vivo and prodisc C Nova products have been in use outside the U.S. since 2009, and prodisc C Vivo is reportedly the most frequently implanted TDR outside of the U.S. prodisc C Vivo product has keel-less endplates including a convex, superior endplate to match more concave vertebral anatomy, while prodisc C SK and prodisc C Nova implant designs have flat endplates with low-profile keels to better match flat vertebral anatomy. All of these products incorporate prodisc CORE technology, the basis behind the predictable clinical outcomes of every prodisc device after 30 years and over 225,000 implantations, worldwide.
Centinel Spine also continues to enroll for a two-level prospective, randomized, multi-centered clinical study evaluating prodisc C Vivo and prodisc C SK.
Centinel Spine’s CEO, Steve Murray, said, “Anatomic cervical total disc options provide surgeons the benefit of selecting implants to optimally fit the disc to each patient. This is unique and represents a major advancement in spinal reconstruction. PMA approval for these three additional devices is a significant accomplishment and we look forward to bringing the new prodisc options to the market in Q4 2022.”
Source: Centinel Spine, LLC

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







