
 Copy to clipboard
Copy to clipboard 
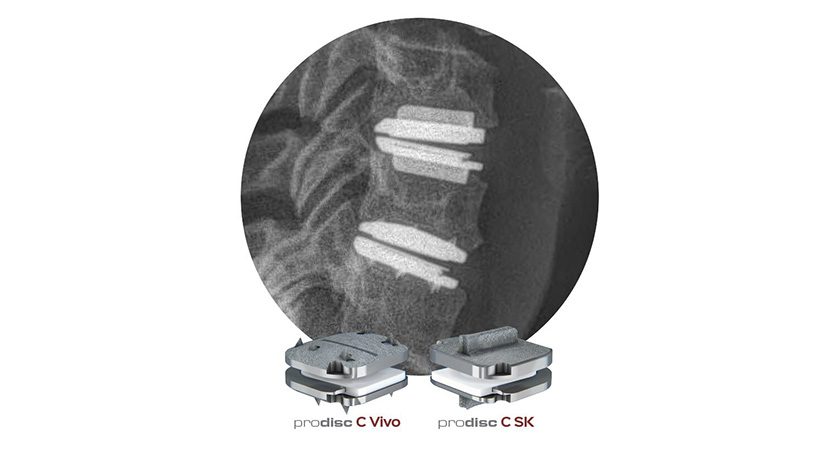
Centinel Spine announced FDA Premarket Approval (PMA) for 2-level indications for the prodiscC Vivo and prodisc C SK Cervical total disc replacement devices. Since receiving FDA approval for 1-level indications in 2022, nearly 20,000 prodisc C Vivo and prodisc C SK spinal levels have been implanted in the U.S. by over 1,100 surgeons.
A total of 480 subjects were enrolled in the Investigational Device Exemption (IDE) Clinical Study across 31 centers, and the PMA is based on the analysis of 433 subjects (the highest number of subjects used to support a PMA for any cervical TDR device on the market).
For study patients who reached the 24-month endpoint, the IDE Clinical Study results demonstrated:
- Patients treated with prodisc C Vivo and prodisc C SK had statistically non-inferior (i.e. equivalent) outcomes versus patients treated with the control cervical TDR device.
- Patients treated with prodisc C Vivo and prodisc C SK achieved an overall composite clinical success rate of 87.1% (vs. 83.7% control cervical TDR), which is the highest overall composite clinical success rate at two levels compared to any other approved cervical TDR device.
- 93.9% of prodisc C Vivo and prodisc C SK patients achieved a meaningful (15 point) improvement in Neck Disability Index vs. 90.1% for the control cervical TDR.
- 96.9% of prodisc C Vivo and prodisc C SK patients had no secondary surgical interventions (revision, removal, re-operation, supplemental fixation) at the index level(s) vs. 95.7% for the control cervical TDR.
Centinel Spine’s CEO, Steve Murray, summed up, “The IDE trial supporting this FDA approval is a landmark study using another FDA-approved disc device as a control and including two different investigational devices that could be used for the same patient. This 2-level approval advances the concept of uniquely matching the disc to each level of the patient’s cervical spine and is a major step forward in total disc replacement.”
Source: Centinel Spine, LLC
Centinel Spine announced FDA Premarket Approval (PMA) for 2-level indications for the prodiscC Vivo and prodisc C SK Cervical total disc replacement devices. Since receiving FDA approval for 1-level indications in 2022, nearly 20,000 prodisc C Vivo and prodisc C SK spinal levels have been implanted in the U.S. by over 1,100 surgeons.
A...
Centinel Spine announced FDA Premarket Approval (PMA) for 2-level indications for the prodiscC Vivo and prodisc C SK Cervical total disc replacement devices. Since receiving FDA approval for 1-level indications in 2022, nearly 20,000 prodisc C Vivo and prodisc C SK spinal levels have been implanted in the U.S. by over 1,100 surgeons.
A total of 480 subjects were enrolled in the Investigational Device Exemption (IDE) Clinical Study across 31 centers, and the PMA is based on the analysis of 433 subjects (the highest number of subjects used to support a PMA for any cervical TDR device on the market).
For study patients who reached the 24-month endpoint, the IDE Clinical Study results demonstrated:
- Patients treated with prodisc C Vivo and prodisc C SK had statistically non-inferior (i.e. equivalent) outcomes versus patients treated with the control cervical TDR device.
- Patients treated with prodisc C Vivo and prodisc C SK achieved an overall composite clinical success rate of 87.1% (vs. 83.7% control cervical TDR), which is the highest overall composite clinical success rate at two levels compared to any other approved cervical TDR device.
- 93.9% of prodisc C Vivo and prodisc C SK patients achieved a meaningful (15 point) improvement in Neck Disability Index vs. 90.1% for the control cervical TDR.
- 96.9% of prodisc C Vivo and prodisc C SK patients had no secondary surgical interventions (revision, removal, re-operation, supplemental fixation) at the index level(s) vs. 95.7% for the control cervical TDR.
Centinel Spine’s CEO, Steve Murray, summed up, “The IDE trial supporting this FDA approval is a landmark study using another FDA-approved disc device as a control and including two different investigational devices that could be used for the same patient. This 2-level approval advances the concept of uniquely matching the disc to each level of the patient’s cervical spine and is a major step forward in total disc replacement.”
Source: Centinel Spine, LLC

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







