Copy to clipboard
Copy to clipboard 
Catalyst OrthoScience was granted FDA 510(k) clearance to market its reverse shoulder system. Limited U.S. launch will begin in 2Q21, followed by commercial launch later this year.
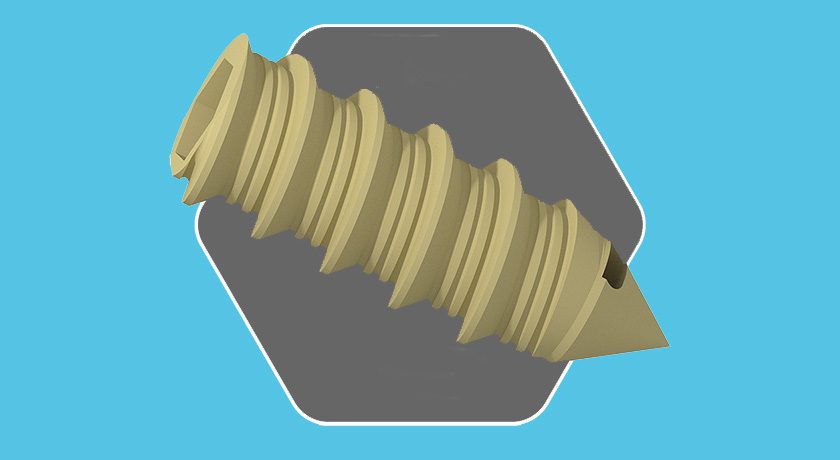
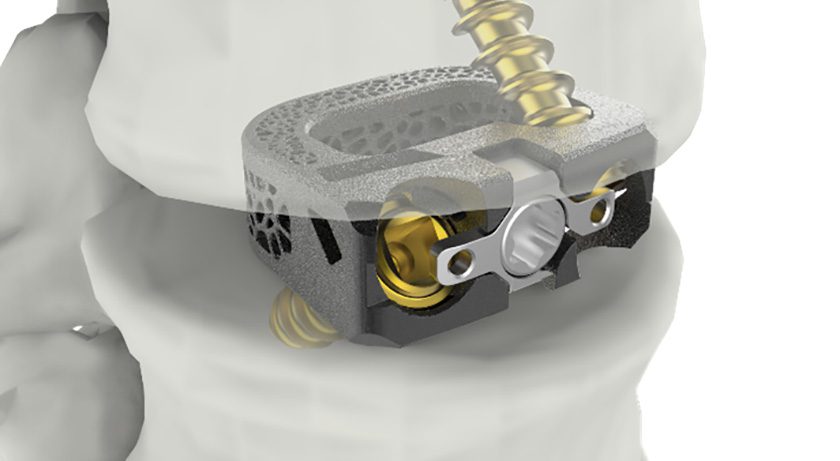
Catalyst’s reverse shoulder system is a single-tray arthroplasty system engineered to combine the most beneficial and evidence-based attributes of reverse shoulder arthroplasty design. The system offers surgeon-targeted implant positioning, a streamlined and versatile system and bone sparing implants.
“With the addition of our reverse shoulder system, Catalyst is now addressing the two fastest growing segments of the shoulder arthroplasty market – stemless anatomic and reverse TSA,” said Brian K. Hutchison, Chairman and CEO of Catalyst.
Catalyst OrthoScience was granted FDA 510(k) clearance to market its reverse shoulder system. Limited U.S. launch will begin in 2Q21, followed by commercial launch later this year.
Catalyst’s reverse shoulder system is a single-tray arthroplasty system engineered to combine the most beneficial and evidence-based attributes of reverse...
Catalyst OrthoScience was granted FDA 510(k) clearance to market its reverse shoulder system. Limited U.S. launch will begin in 2Q21, followed by commercial launch later this year.
Catalyst’s reverse shoulder system is a single-tray arthroplasty system engineered to combine the most beneficial and evidence-based attributes of reverse shoulder arthroplasty design. The system offers surgeon-targeted implant positioning, a streamlined and versatile system and bone sparing implants.
“With the addition of our reverse shoulder system, Catalyst is now addressing the two fastest growing segments of the shoulder arthroplasty market – stemless anatomic and reverse TSA,” said Brian K. Hutchison, Chairman and CEO of Catalyst.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.