





Catalyst OrthoScience raised $12.3 million in an oversubscribed Series D financing round.
Thus far in 2021, Catalyst has:
- Rebranded its product portfolio with the Archer family name,
- Received FDA 510(k) clearance and began a limited launch for the Archer R1 Reverse Shoulder System and
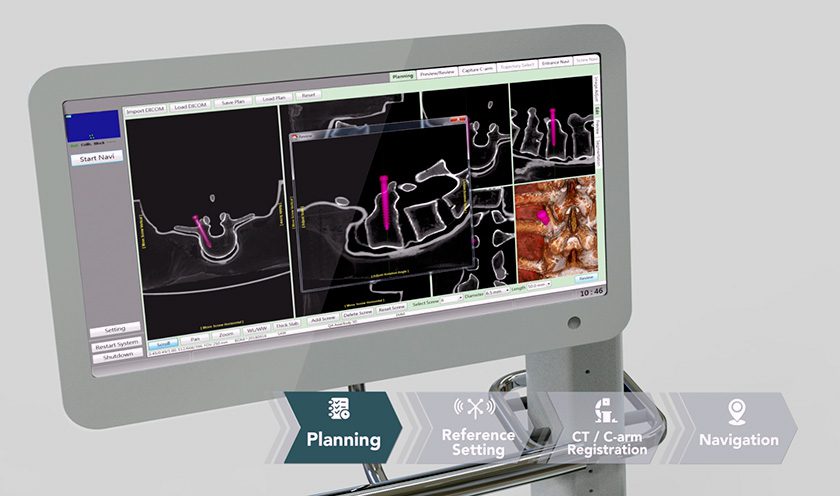
- Introduced its Archer 3D Targeting imaging software for its current product offerings.
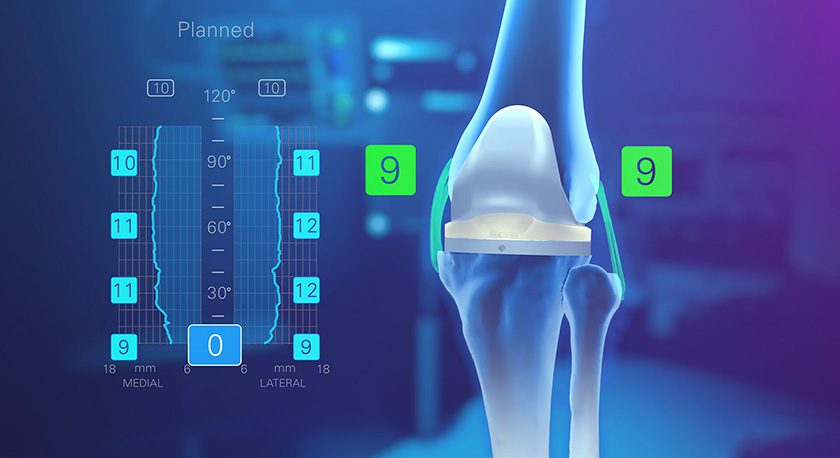
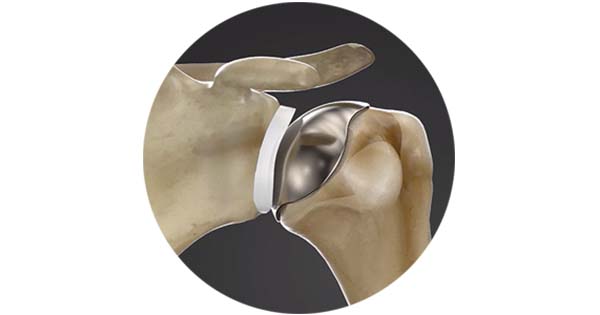
Additionally, the Archer CSR Total Shoulder System, Catalyst’s stemless, ellipsoid anatomic system, has continued rapid adoption and growth. Today, the Archer shoulder systems have been used in more than 3,000 surgeries. The first patients are more than five years post-surgery with excellent clinical results.
“This funding round comes at a pivotal time for the Catalyst OrthoScience team,” said Brian K. Hutchison, chairman and CEO of Catalyst. “We are experiencing a significant period of growth, and this funding allows us to enhance our innovative product portfolio with further technological advancements in the shoulder arthroplasty space and expand our team across all areas.”
Source: Catalyst OrthoScience Inc.
Catalyst OrthoScience raised $12.3 million in an oversubscribed Series D financing round.
Thus far in 2021, Catalyst has:
Rebranded its product portfolio with the Archer family name,
Received FDA 510(k) clearance and began a limited launch for the Archer R1 Reverse Shoulder System and
Introduced its Archer 3D Targeting...
Catalyst OrthoScience raised $12.3 million in an oversubscribed Series D financing round.
Thus far in 2021, Catalyst has:
- Rebranded its product portfolio with the Archer family name,
- Received FDA 510(k) clearance and began a limited launch for the Archer R1 Reverse Shoulder System and
- Introduced its Archer 3D Targeting imaging software for its current product offerings.
Additionally, the Archer CSR Total Shoulder System, Catalyst’s stemless, ellipsoid anatomic system, has continued rapid adoption and growth. Today, the Archer shoulder systems have been used in more than 3,000 surgeries. The first patients are more than five years post-surgery with excellent clinical results.
“This funding round comes at a pivotal time for the Catalyst OrthoScience team,” said Brian K. Hutchison, chairman and CEO of Catalyst. “We are experiencing a significant period of growth, and this funding allows us to enhance our innovative product portfolio with further technological advancements in the shoulder arthroplasty space and expand our team across all areas.”
Source: Catalyst OrthoScience Inc.


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.