
 Copy to clipboard
Copy to clipboard 
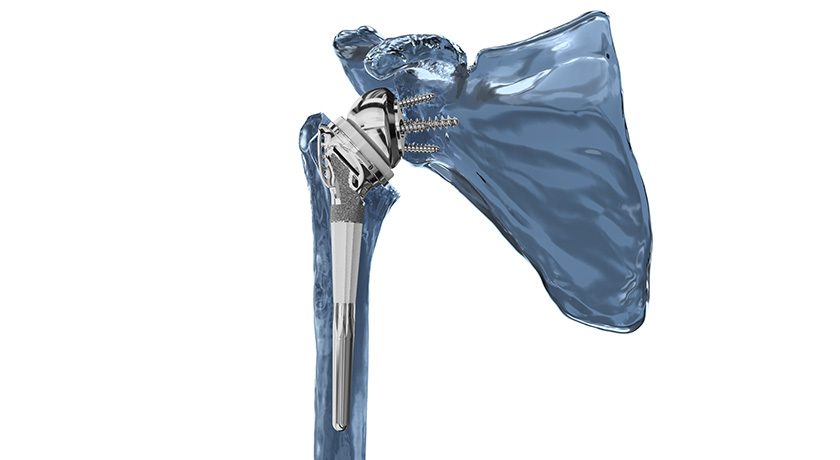
Catalyst OrthoScience announced FDA 510(k) clearance for and first surgeries with the Catalyst Fracture Shoulder System, a reverse arthroplasty system to treat proximal humeral fractures.
Key elements of the Catalyst Fracture System for reverse shoulder replacement include:
- Tuberosity-specific fossa designed to maximize tuberosity contact and healing to a porous surface.
- Patented tuberosity retention rails designed to further restrict tuberosity motion post-operatively.
- Uncemented, secure press-fit diaphyseal fixation and strong rotational stability, with cemented options as well.
- Compatibility with glenospheres and baseplates in standard, augmented, and lateralized configurations to suit nearly every type of glenoid anatomy.
Catalyst will complete a limited user release to gather further data on the system’s performance ahead of a broader commercial release in 2025.
“With the addition of the Catalyst Fracture System, we now have a comprehensive portfolio that allows surgeons to help a broader range of patients,” said Carl O’Connell, CEO of Catalyst. “This product is key to our strategy and builds our leadership in the orthopedics market.”
Source: Catalyst OrthoScience Inc.
Catalyst OrthoScience announced FDA 510(k) clearance for and first surgeries with the Catalyst Fracture Shoulder System, a reverse arthroplasty system to treat proximal humeral fractures.
Key elements of the Catalyst Fracture System for reverse shoulder replacement include:
Tuberosity-specific fossa designed to maximize tuberosity contact...
Catalyst OrthoScience announced FDA 510(k) clearance for and first surgeries with the Catalyst Fracture Shoulder System, a reverse arthroplasty system to treat proximal humeral fractures.
Key elements of the Catalyst Fracture System for reverse shoulder replacement include:
- Tuberosity-specific fossa designed to maximize tuberosity contact and healing to a porous surface.
- Patented tuberosity retention rails designed to further restrict tuberosity motion post-operatively.
- Uncemented, secure press-fit diaphyseal fixation and strong rotational stability, with cemented options as well.
- Compatibility with glenospheres and baseplates in standard, augmented, and lateralized configurations to suit nearly every type of glenoid anatomy.
Catalyst will complete a limited user release to gather further data on the system’s performance ahead of a broader commercial release in 2025.
“With the addition of the Catalyst Fracture System, we now have a comprehensive portfolio that allows surgeons to help a broader range of patients,” said Carl O’Connell, CEO of Catalyst. “This product is key to our strategy and builds our leadership in the orthopedics market.”
Source: Catalyst OrthoScience Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







