Copy to clipboard
Copy to clipboard 
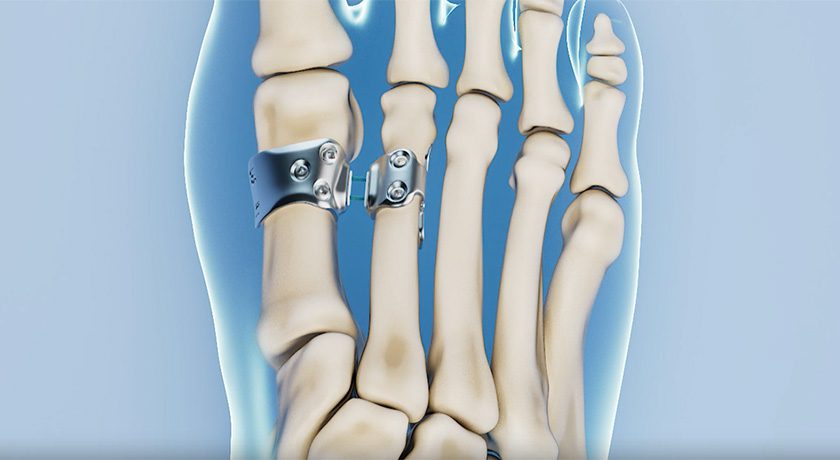
Cartiva secured US $8.5MM in an oversubscribed Series E financing. Proceeds will support commercialization of the Synthetic Cartilage Implant (SCI) for treatment of great toe arthritis and general corporate purposes.
Cartiva received FDA Premarket Approval for SCI in early 3Q16, representing what is reported to be the first synthetic cartilage device to gain FDA approval. The first commercial U.S. patient received the implant shortly thereafter.
The financing was led by New Enterprise Associates with participation by Windham Venture Partners and returning investors.
Sources: Cartiva, Inc.; ORTHOWORLD Inc.
Cartiva secured US $8.5MM in an oversubscribed Series E financing. Proceeds will support commercialization of the Synthetic Cartilage Implant (SCI) for treatment of great toe arthritis and general corporate purposes.
Cartiva received FDA Premarket Approval for SCI in early 3Q16, representing what is reported to be the first synthetic cartilage...
Cartiva secured US $8.5MM in an oversubscribed Series E financing. Proceeds will support commercialization of the Synthetic Cartilage Implant (SCI) for treatment of great toe arthritis and general corporate purposes.
Cartiva received FDA Premarket Approval for SCI in early 3Q16, representing what is reported to be the first synthetic cartilage device to gain FDA approval. The first commercial U.S. patient received the implant shortly thereafter.
The financing was led by New Enterprise Associates with participation by Windham Venture Partners and returning investors.
Sources: Cartiva, Inc.; ORTHOWORLD Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.