Copy to clipboard
Copy to clipboard 
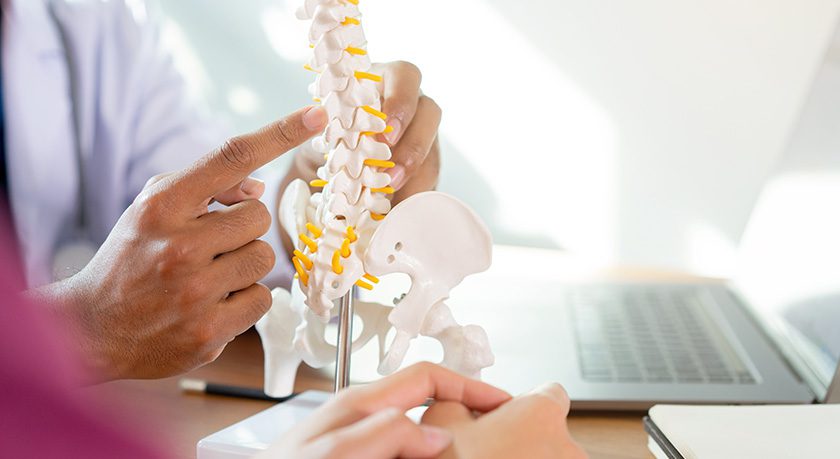
The first procedure has been performed using Carlsmed’s aprevo patient specific spinal implant. Carlsmed uses data to create spine fusion devices that are specifically designed and manufactured for each patient.
The Carlsmed aprevo device is the first implant to receive both Breakthrough Device Designation and 510(k) market clearance from FDA.
Mike Cordonnier, CEO of Carlsmed, said, “With the advent of Carlsmed’s digital to device technology, there is no longer a need for hospitals to manage the complex inventory logistics of traditional surgical implants. The aprevo family of patient specific devices eliminates the tedious and costly process of stock device inventory control and reprocessing.”
The first procedure has been performed using Carlsmed's aprevo patient specific spinal implant. Carlsmed uses data to create spine fusion devices that are specifically designed and manufactured for each patient.
The Carlsmed aprevo device is the first implant to receive both Breakthrough Device Designation and 510(k) market...
The first procedure has been performed using Carlsmed’s aprevo patient specific spinal implant. Carlsmed uses data to create spine fusion devices that are specifically designed and manufactured for each patient.
The Carlsmed aprevo device is the first implant to receive both Breakthrough Device Designation and 510(k) market clearance from FDA.
Mike Cordonnier, CEO of Carlsmed, said, “With the advent of Carlsmed’s digital to device technology, there is no longer a need for hospitals to manage the complex inventory logistics of traditional surgical implants. The aprevo family of patient specific devices eliminates the tedious and costly process of stock device inventory control and reprocessing.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.