
 Copy to clipboard
Copy to clipboard 
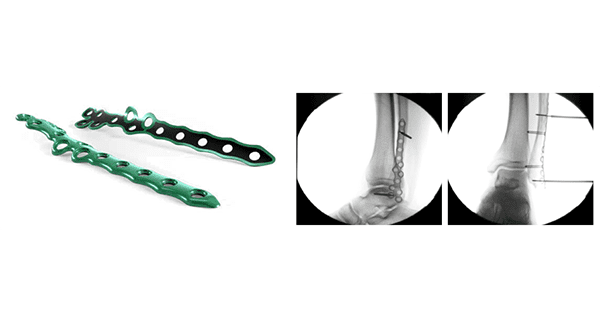
Carbon22, a GLW, Inc. medical technology company, received FDA 510(k) clearance to market the Apollo Ankle Fracture Plating System, a novel portfolio of see-through, “ortholucent” bone plates and screws used for orthopedic ankle fracture surgery.
Expected to commercially launch in late spring 2022, Apollo will complement Carbon22’s Creed Ortholucent Implant portfolio of headless and headed compression screws.
The streamlined, multi-component platform features a proprietary plate composite consisting of an additive manufactured titanium shell injection molded with a novel Solvay Zeniva® PEEK polymer. The implants come with a selection of 26 unique plate options from 6 plate families.
Carbon22’s patent-pending ortholucent manufacturing technology is designed to offer several benefits, including radio-transparency.
The hybrid titanium/PEEK plate interface minimizes the risk of plate and screw ”cold-welding” that has been seen with other plating systems currently on the market. Additionally, the hybrid titanium/PEEK construction of the plates allow for contouring to accommodate complex anatomy.
Source: Carbon22, a GLW Inc. Company
Carbon22, a GLW, Inc. medical technology company, received FDA 510(k) clearance to market the Apollo Ankle Fracture Plating System, a novel portfolio of see-through, “ortholucent” bone plates and screws used for orthopedic ankle fracture surgery.
Expected to commercially launch in late spring 2022, Apollo will complement Carbon22’s Creed...
Carbon22, a GLW, Inc. medical technology company, received FDA 510(k) clearance to market the Apollo Ankle Fracture Plating System, a novel portfolio of see-through, “ortholucent” bone plates and screws used for orthopedic ankle fracture surgery.
Expected to commercially launch in late spring 2022, Apollo will complement Carbon22’s Creed Ortholucent Implant portfolio of headless and headed compression screws.
The streamlined, multi-component platform features a proprietary plate composite consisting of an additive manufactured titanium shell injection molded with a novel Solvay Zeniva® PEEK polymer. The implants come with a selection of 26 unique plate options from 6 plate families.
Carbon22’s patent-pending ortholucent manufacturing technology is designed to offer several benefits, including radio-transparency.
The hybrid titanium/PEEK plate interface minimizes the risk of plate and screw ”cold-welding” that has been seen with other plating systems currently on the market. Additionally, the hybrid titanium/PEEK construction of the plates allow for contouring to accommodate complex anatomy.
Source: Carbon22, a GLW Inc. Company

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.