
 Copy to clipboard
Copy to clipboard 
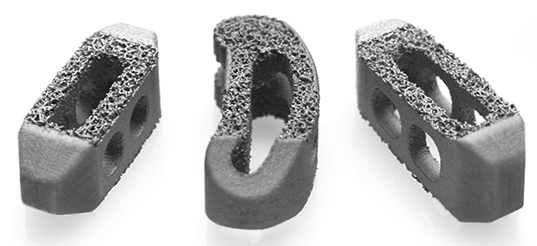 Captiva Spine received FDA 510(k) clearance to market TirboLOX-L, a titanium lumbar cage featuring a dual-layer lattice structure with a micro-rough surface, dual porosity and open architecture.
Captiva Spine received FDA 510(k) clearance to market TirboLOX-L, a titanium lumbar cage featuring a dual-layer lattice structure with a micro-rough surface, dual porosity and open architecture.
TirboLOX-L’s structure is designed to reduce radiographic presence for clear imaging, and its high coefficient of friction supports immediate bidirectional fixation, according to the company.
The device incorporates Captiva’s Pivotec® Pivoting TLIF cage, which is designed to addresschallenges of controlling cage insertion and angle manipulation during minimally invasive procedures.
Source: Captiva Spine; Image courtesy of Captiva Spine
Captiva Spine received FDA 510(k) clearance to market TirboLOX-L, a titanium lumbar cage featuring a dual-layer lattice structure with a micro-rough surface, dual porosity and open architecture.
TirboLOX-L’s structure is designed to reduce radiographic presence for clear imaging, and its high coefficient of friction supports immediate...
 Captiva Spine received FDA 510(k) clearance to market TirboLOX-L, a titanium lumbar cage featuring a dual-layer lattice structure with a micro-rough surface, dual porosity and open architecture.
Captiva Spine received FDA 510(k) clearance to market TirboLOX-L, a titanium lumbar cage featuring a dual-layer lattice structure with a micro-rough surface, dual porosity and open architecture.
TirboLOX-L’s structure is designed to reduce radiographic presence for clear imaging, and its high coefficient of friction supports immediate bidirectional fixation, according to the company.
The device incorporates Captiva’s Pivotec® Pivoting TLIF cage, which is designed to addresschallenges of controlling cage insertion and angle manipulation during minimally invasive procedures.
Source: Captiva Spine; Image courtesy of Captiva Spine

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







