
 Copy to clipboard
Copy to clipboard 
Camber Spine announced the successful completion of first surgeries with the SPIRA™-C Open Matrix cervical interbody fusion implant.
The device, which received FDA 510(k) clearance in late 4Q17, features 3D-printed spiral support arches with large openings to accept a significant amount of graft and distribute load-sharing. Proprietary Surface by Design™ technology is intended to increase fusion rates and stabilization.
Source: Camber Spine Technologies, LLP
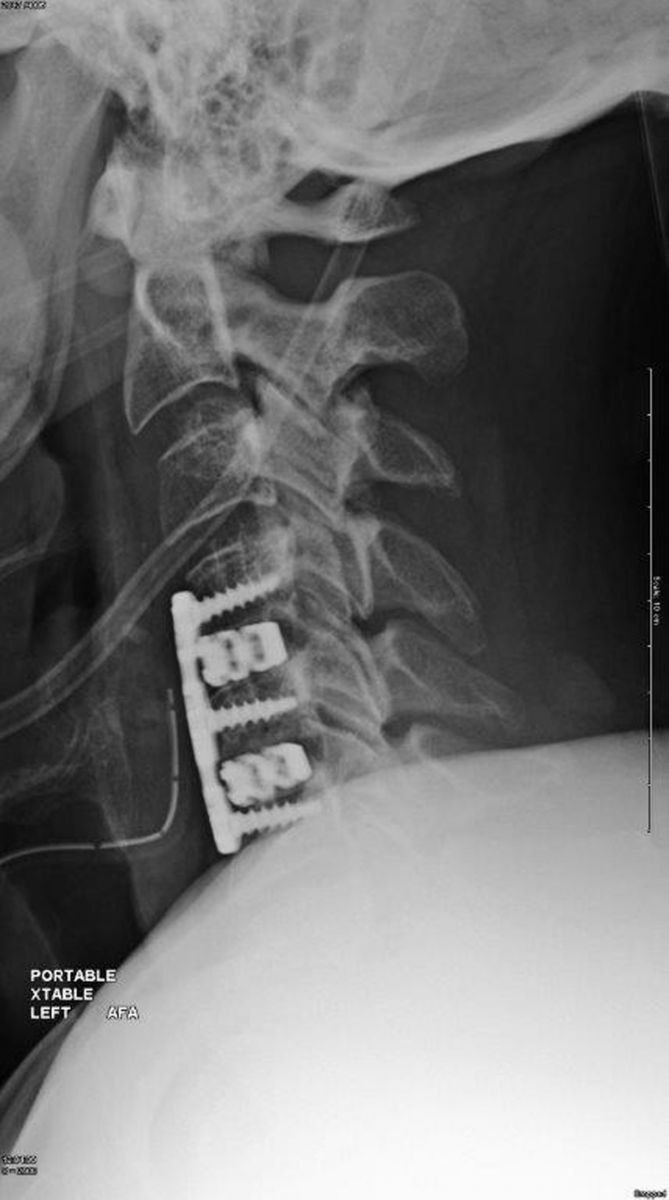
Image courtesy of Camber Spine Technologies
Camber Spine announced the successful completion of first surgeries with the SPIRA™-C Open Matrix cervical interbody fusion implant.
The device, which received FDA 510(k) clearance in late 4Q17, features 3D-printed spiral support arches with large openings to accept a significant amount of graft and distribute load-sharing. Proprietary Surface...
Camber Spine announced the successful completion of first surgeries with the SPIRA™-C Open Matrix cervical interbody fusion implant.
The device, which received FDA 510(k) clearance in late 4Q17, features 3D-printed spiral support arches with large openings to accept a significant amount of graft and distribute load-sharing. Proprietary Surface by Design™ technology is intended to increase fusion rates and stabilization.
Source: Camber Spine Technologies, LLP

Image courtesy of Camber Spine Technologies

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







