
 Copy to clipboard
Copy to clipboard 
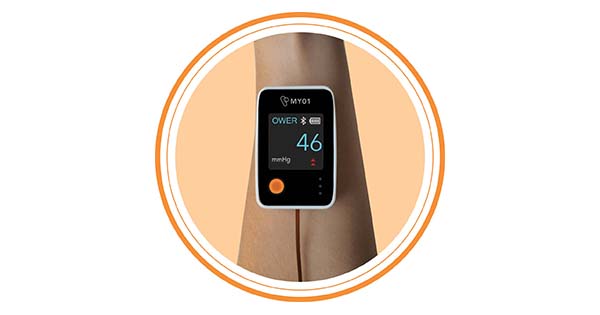
The MY01 Continuous Compartmental Pressure Monitor provides reliable, real-time continuous pressure measurements to aid in the diagnosis of compartment syndrome. The receipt of Breakthrough Device Designation demonstrates that MY01 can offer significant advantages over existing alternatives for more effective and efficient management of Compartment Syndrome.
AAOS, the American Academy of Orthopaedic Surgeons, recognizes the use of continuous pressure monitoring to aid in the diagnosis of Compartment Syndrome. Compartment syndrome is a potentially devastating and relatively common complication of fractures of the knee and tibial shaft.
While clinical assessment alone or single-point measurements can lead to delayed diagnosis or overtreatment, continuous IntraCompartmental Pressure augments early decision-making in Acute Compartment Syndrome (ACS). Optimal management of ACS depends on early diagnosis and intervention, which significantly reduces a patient’s chance of amputation, infection and nerve damage, as well as minimizing the need for unnecessary fasciotomies.
The MY01 Continuous Compartmental Pressure Monitor (and its companion mobile app) received FDA 510(k) clearance earlier this year and is being used in hospitals around the U.S. to aid in the timely diagnosis of Compartment Syndrome. The simple, single-use device utilizes proprietary technology to deliver a microsensor directly to the at-risk muscle compartment to continuously measure pressure for up to 18 hours. Pressure readings are wirelessly transmitted to the MY01 app and stored on the Cloud, enabling true care team collaboration for more efficient patient care.
Source: MY01
The MY01 Continuous Compartmental Pressure Monitor provides reliable, real-time continuous pressure measurements to aid in the diagnosis of compartment syndrome. The receipt of Breakthrough Device Designation demonstrates that MY01 can offer significant advantages over existing alternatives for more effective and efficient management of...
The MY01 Continuous Compartmental Pressure Monitor provides reliable, real-time continuous pressure measurements to aid in the diagnosis of compartment syndrome. The receipt of Breakthrough Device Designation demonstrates that MY01 can offer significant advantages over existing alternatives for more effective and efficient management of Compartment Syndrome.
AAOS, the American Academy of Orthopaedic Surgeons, recognizes the use of continuous pressure monitoring to aid in the diagnosis of Compartment Syndrome. Compartment syndrome is a potentially devastating and relatively common complication of fractures of the knee and tibial shaft.
While clinical assessment alone or single-point measurements can lead to delayed diagnosis or overtreatment, continuous IntraCompartmental Pressure augments early decision-making in Acute Compartment Syndrome (ACS). Optimal management of ACS depends on early diagnosis and intervention, which significantly reduces a patient’s chance of amputation, infection and nerve damage, as well as minimizing the need for unnecessary fasciotomies.
The MY01 Continuous Compartmental Pressure Monitor (and its companion mobile app) received FDA 510(k) clearance earlier this year and is being used in hospitals around the U.S. to aid in the timely diagnosis of Compartment Syndrome. The simple, single-use device utilizes proprietary technology to deliver a microsensor directly to the at-risk muscle compartment to continuously measure pressure for up to 18 hours. Pressure readings are wirelessly transmitted to the MY01 app and stored on the Cloud, enabling true care team collaboration for more efficient patient care.
Source: MY01

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







