
 Copy to clipboard
Copy to clipboard 
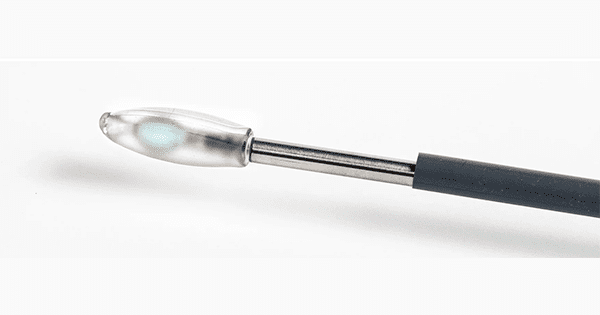
Spinal Stabilization Technologies (SST) earned CE Mark approval and FDA Breakthrough Designation for the PerQdisc™ Nucleus Replacement.
PerQdisc is reportedly the only commercially available lumbar nucleus replacement system in the world. The device replaces the nucleus pulposus of the intervertebral disc in the L1 – S1 spinal region in patients with single-level discogenic pain. The patient may have single or multi-level degenerative disc disease (DDD), but the discogenic pain must be limited to a single level.
The PerQdisc procedure offers an alternative to surgical treatment with spinal fusion or total disc replacement. A small incision minimizes the risk of blood loss and results in a custom implant that fills the patient’s disc space.
The device and surgical procedure are not yet FDA cleared. Mark Novotny, SST’s CEO, said, “SST is working with the FDA to develop a comprehensive clinical trial program. As part of that process, the FDA recently designated the PerQdisc as ‘breakthrough technology.’ In the meantime, with the CE Mark approval, SST has plans to launch sales of its device in Europe, the Middle East, and Asia including China.”
Spinal Stabilization Technologies (SST) earned CE Mark approval and FDA Breakthrough Designation for the PerQdisc™ Nucleus Replacement.
PerQdisc is reportedly the only commercially available lumbar nucleus replacement system in the world. The device replaces the nucleus pulposus of the intervertebral disc in the L1 - S1 spinal region in...
Spinal Stabilization Technologies (SST) earned CE Mark approval and FDA Breakthrough Designation for the PerQdisc™ Nucleus Replacement.
PerQdisc is reportedly the only commercially available lumbar nucleus replacement system in the world. The device replaces the nucleus pulposus of the intervertebral disc in the L1 – S1 spinal region in patients with single-level discogenic pain. The patient may have single or multi-level degenerative disc disease (DDD), but the discogenic pain must be limited to a single level.
The PerQdisc procedure offers an alternative to surgical treatment with spinal fusion or total disc replacement. A small incision minimizes the risk of blood loss and results in a custom implant that fills the patient’s disc space.
The device and surgical procedure are not yet FDA cleared. Mark Novotny, SST’s CEO, said, “SST is working with the FDA to develop a comprehensive clinical trial program. As part of that process, the FDA recently designated the PerQdisc as ‘breakthrough technology.’ In the meantime, with the CE Mark approval, SST has plans to launch sales of its device in Europe, the Middle East, and Asia including China.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







