
 Copy to clipboard
Copy to clipboard 
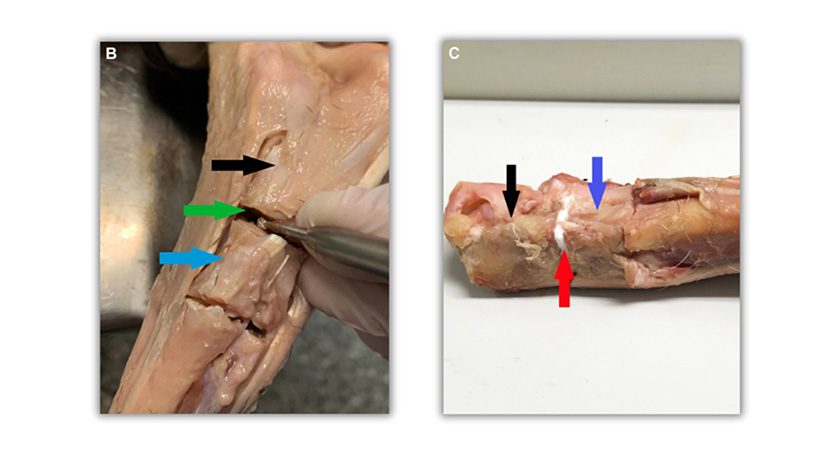
Biomimetic Innovations’ OsStic was granted Breakthrough Device designation by FDA.
The proposed indication statement for this novel new technology is, “OsStic Synthetic Injectable Bone Void Filler is a structural, mechanically enhanced bioadhesive for reduction, provisional fixation or void filling of peri-articular fractures or defects to enhance structural stability where standard fixation alone cannot provide sufficient support for functional mobilization.”
OsStic is designed to evolve structural orthobiologics to the point where surgeons can now use this material to aid the reduction, provisional fixation and void filling of peri-articular fractures. This is reported to be the first calcium phosphate that meets all these clinical requirements.
Source: Biomimetic Innovations Ltd
Biomimetic Innovations' OsStic was granted Breakthrough Device designation by FDA.
The proposed indication statement for this novel new technology is, “OsStic Synthetic Injectable Bone Void Filler is a structural, mechanically enhanced bioadhesive for reduction, provisional fixation or void filling of peri-articular fractures or defects to...
Biomimetic Innovations’ OsStic was granted Breakthrough Device designation by FDA.
The proposed indication statement for this novel new technology is, “OsStic Synthetic Injectable Bone Void Filler is a structural, mechanically enhanced bioadhesive for reduction, provisional fixation or void filling of peri-articular fractures or defects to enhance structural stability where standard fixation alone cannot provide sufficient support for functional mobilization.”
OsStic is designed to evolve structural orthobiologics to the point where surgeons can now use this material to aid the reduction, provisional fixation and void filling of peri-articular fractures. This is reported to be the first calcium phosphate that meets all these clinical requirements.
Source: Biomimetic Innovations Ltd

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







