Copy to clipboard
Copy to clipboard 
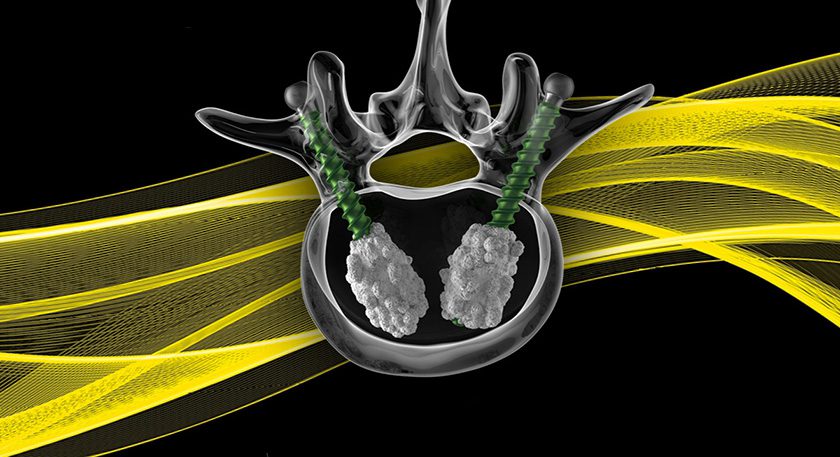
FDA granted expanded clearance for Bone Solutions’ Mg OSTEOCRETE and Mg OSTEOINJECT products, allowing for use of the technologies in pediatric patients aged six years and older.
This indication highlights the adaptability of Bone Solutions’ proprietary magnesium-based platform, particularly in the Sclerograft procedure—a minimally invasive outpatient technique for treating unicameral bone cysts. The procedure involves chemical curettage of the cyst wall with doxycycline, followed by injection of Mg OSTEOINJECT low-viscosity bone graft substitute. Mg OSTEOINJECT is designed to fill bone voids or defects in the extremities or pelvis resulting from tumor or cyst removal, trauma, or subchondral cystic changes due to osteoarthritis.
Mg OSTEOCRETE and Mg OSTEOINJECT are magnesium oxide-based formulations that initiate a crystallization reaction with phosphate compounds to deliver optimal osteoconductivity. This promotes osteoblast activity, including cell adhesion, proliferation, and extracellular matrix formation to support bone regeneration.
The products are drillable and offer exceptional handling properties. After less than a minute of mixing, the material becomes moldable or injectable, demonstrating cohesive adhesion and radiopacity.
“We’re thrilled to bring this innovative, minimally invasive solution to the pediatric market,” said Drew Diaz, CEO of Bone Solutions Inc. “This clearance means children can receive effective treatment and return home faster than with traditional methods. Our mission remains focused on enhancing patient outcomes through advanced magnesium-based orthopedic technologies.”
Source: Bone Solutions Inc.
FDA granted expanded clearance for Bone Solutions' Mg OSTEOCRETE and Mg OSTEOINJECT products, allowing for use of the technologies in pediatric patients aged six years and older.
This indication highlights the adaptability of Bone Solutions’ proprietary magnesium-based platform, particularly in the Sclerograft procedure—a minimally invasive...
FDA granted expanded clearance for Bone Solutions’ Mg OSTEOCRETE and Mg OSTEOINJECT products, allowing for use of the technologies in pediatric patients aged six years and older.
This indication highlights the adaptability of Bone Solutions’ proprietary magnesium-based platform, particularly in the Sclerograft procedure—a minimally invasive outpatient technique for treating unicameral bone cysts. The procedure involves chemical curettage of the cyst wall with doxycycline, followed by injection of Mg OSTEOINJECT low-viscosity bone graft substitute. Mg OSTEOINJECT is designed to fill bone voids or defects in the extremities or pelvis resulting from tumor or cyst removal, trauma, or subchondral cystic changes due to osteoarthritis.
Mg OSTEOCRETE and Mg OSTEOINJECT are magnesium oxide-based formulations that initiate a crystallization reaction with phosphate compounds to deliver optimal osteoconductivity. This promotes osteoblast activity, including cell adhesion, proliferation, and extracellular matrix formation to support bone regeneration.
The products are drillable and offer exceptional handling properties. After less than a minute of mixing, the material becomes moldable or injectable, demonstrating cohesive adhesion and radiopacity.
“We’re thrilled to bring this innovative, minimally invasive solution to the pediatric market,” said Drew Diaz, CEO of Bone Solutions Inc. “This clearance means children can receive effective treatment and return home faster than with traditional methods. Our mission remains focused on enhancing patient outcomes through advanced magnesium-based orthopedic technologies.”
Source: Bone Solutions Inc.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.