
 Copy to clipboard
Copy to clipboard 
Circinus Medical Technology received FDA 510(k) clearance to market its novel Bolt Navigation System.
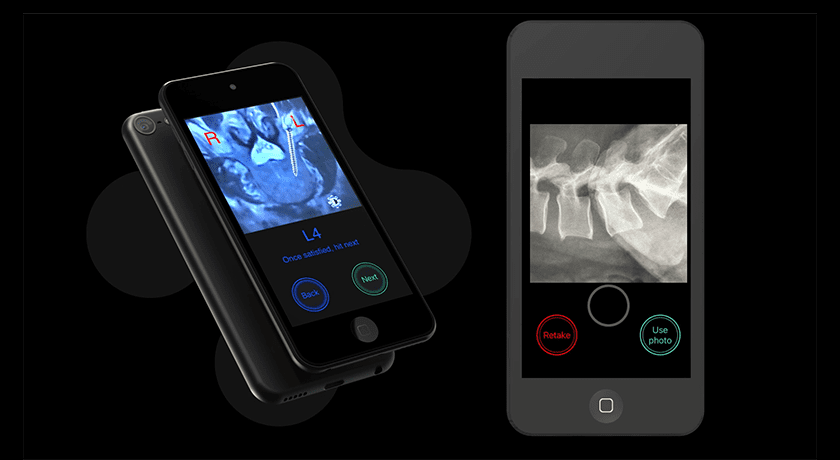
Bolt Navigation is designed to provide an accurate, easy to use, economic spine navigation solution. It utilizes an iOS device, with a high-powered processor, familiar user interface and highly sensitive gyroscope-on-chip™ technology to support efficient and effective placement of posterior spine fixation. Its near-zero footprint, instrument-agnostic design, ability to utilize standard imaging and subscription business model make it suitable for today’s evolving surgical market.
In late 2021, the company secured funding to support commercialization of Bolt Navigation in the U.S., Europe and select emerging markets; continued expansion of its portfolio of applications; development of additional disruptive solutions and growth of intellectual property.
“We are pleased to have received this approval,” said John Dorman, MD, the company’s Founder. “A great deal of hard work by the Bolt team has allowed us to recognize my vision of making powerful surgical technology more broadly available.”
Patrick West, CEO, added, “While it is undoubtedly an exciting and essential milestone for the company, it is in fact an acknowledgement that we have only made it to the starting line. The real work begins now to achieve our goal of allowing any patient, anywhere in the world to benefit from best-in-class surgical technology.”
Source: Circinus Medical Technology
Circinus Medical Technology received FDA 510(k) clearance to market its novel Bolt Navigation System.
Bolt Navigation is designed to provide an accurate, easy to use, economic spine navigation solution. It utilizes an iOS device, with a high-powered processor, familiar user interface and highly sensitive gyroscope-on-chip™ technology to...
Circinus Medical Technology received FDA 510(k) clearance to market its novel Bolt Navigation System.
Bolt Navigation is designed to provide an accurate, easy to use, economic spine navigation solution. It utilizes an iOS device, with a high-powered processor, familiar user interface and highly sensitive gyroscope-on-chip™ technology to support efficient and effective placement of posterior spine fixation. Its near-zero footprint, instrument-agnostic design, ability to utilize standard imaging and subscription business model make it suitable for today’s evolving surgical market.
In late 2021, the company secured funding to support commercialization of Bolt Navigation in the U.S., Europe and select emerging markets; continued expansion of its portfolio of applications; development of additional disruptive solutions and growth of intellectual property.
“We are pleased to have received this approval,” said John Dorman, MD, the company’s Founder. “A great deal of hard work by the Bolt team has allowed us to recognize my vision of making powerful surgical technology more broadly available.”
Patrick West, CEO, added, “While it is undoubtedly an exciting and essential milestone for the company, it is in fact an acknowledgement that we have only made it to the starting line. The real work begins now to achieve our goal of allowing any patient, anywhere in the world to benefit from best-in-class surgical technology.”
Source: Circinus Medical Technology

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







