

 Copy to clipboard
Copy to clipboard 
Bodycad was granted FDA 510(k) clearance for BC Fine Osteotomy™, the first personalized planning and plating system for osteotomies around the knee.
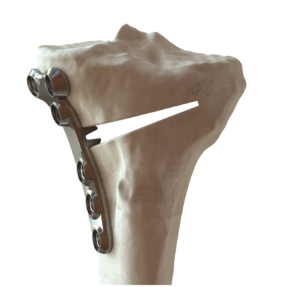
Bodycad BC Fine Osteotomy
BC Fine Osteotomy, similar to Bodycad proprietary software, offers a procedural solution adapted to each patient. It comprises a surgeon-approved 3D-printed patient-specific surgical guide and patient-specific plate with a small titanium wedge to confirm the 3D angular correction.
Bodycad BC Fine Osteotomy includes calibrated drill guides with positive stops to protect posterior neurovascular structures, multi-planar 3D planning to increase accuracy and is delivered as a procedure in a box to drive efficiency in the O.R.
Bodycad was granted FDA 510(k) clearance for BC Fine Osteotomy™, the first personalized planning and plating system for osteotomies around the knee.
BC Fine Osteotomy, similar to Bodycad proprietary software, offers a procedural solution adapted to each patient. It comprises a surgeon-approved 3D-printed patient-specific surgical guide and...
Bodycad was granted FDA 510(k) clearance for BC Fine Osteotomy™, the first personalized planning and plating system for osteotomies around the knee.

Bodycad BC Fine Osteotomy
BC Fine Osteotomy, similar to Bodycad proprietary software, offers a procedural solution adapted to each patient. It comprises a surgeon-approved 3D-printed patient-specific surgical guide and patient-specific plate with a small titanium wedge to confirm the 3D angular correction.
Bodycad BC Fine Osteotomy includes calibrated drill guides with positive stops to protect posterior neurovascular structures, multi-planar 3D planning to increase accuracy and is delivered as a procedure in a box to drive efficiency in the O.R.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







