
 Copy to clipboard
Copy to clipboard 
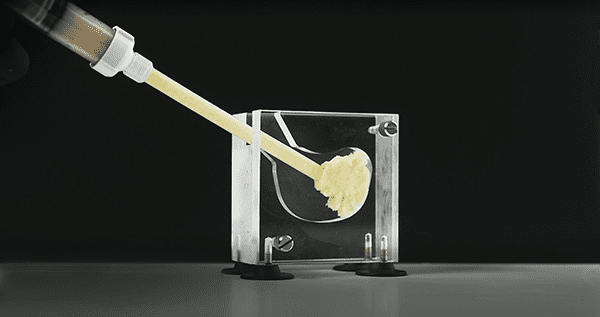
Bioventus commenced U.S. launch of OSTEOAMP® SELECT Flowable, an allograft bone graft substitute developed for procedures including lumbar spine fusion, cervical spine fusion and foot & ankle fusion.
OSTEOAMP SELECT Flowable comes in three sizes from 2.5 to 10 cc and is available nationwide.
Introduced in a limited release in select U.S. markets beginning in March 2021, OSTEOAMP SELECT Flowable is 100% allograft with no synthetic carrier added, yet still based on the OSTEOAMP process designed to retain a wide array of essential growth factors. Its versatile handling is designed to satisfy the need for a flowable allograft product with cohesive properties, making it an attractive option for minimally invasive surgery, expandable cages and 3D-printed cages.
“Spine, trauma and foot & ankle surgeons are looking for allograft options that handle well for a variety of procedures,” said Dr. Larry Boyd, Vice President, Product Development, Bioventus. “Developed by our team in collaboration with our tissue bank partner, OSTEOAMP SELECT Flowable comes ready-to-use, and is designed to be delivered in a range of methods, and to provide excellent retention characteristics at the grafting site. It is terminally sterilized and processed using advanced procedures designed to comply with the highest standards for tissue banking, including comprehensive donor screening and extensive microbiological testing.”
Source: Bioventus
Bioventus commenced U.S. launch of OSTEOAMP® SELECT Flowable, an allograft bone graft substitute developed for procedures including lumbar spine fusion, cervical spine fusion and foot & ankle fusion.
OSTEOAMP SELECT Flowable comes in three sizes from 2.5 to 10 cc and is available nationwide.
Introduced in a limited release in select...
Bioventus commenced U.S. launch of OSTEOAMP® SELECT Flowable, an allograft bone graft substitute developed for procedures including lumbar spine fusion, cervical spine fusion and foot & ankle fusion.
OSTEOAMP SELECT Flowable comes in three sizes from 2.5 to 10 cc and is available nationwide.
Introduced in a limited release in select U.S. markets beginning in March 2021, OSTEOAMP SELECT Flowable is 100% allograft with no synthetic carrier added, yet still based on the OSTEOAMP process designed to retain a wide array of essential growth factors. Its versatile handling is designed to satisfy the need for a flowable allograft product with cohesive properties, making it an attractive option for minimally invasive surgery, expandable cages and 3D-printed cages.
“Spine, trauma and foot & ankle surgeons are looking for allograft options that handle well for a variety of procedures,” said Dr. Larry Boyd, Vice President, Product Development, Bioventus. “Developed by our team in collaboration with our tissue bank partner, OSTEOAMP SELECT Flowable comes ready-to-use, and is designed to be delivered in a range of methods, and to provide excellent retention characteristics at the grafting site. It is terminally sterilized and processed using advanced procedures designed to comply with the highest standards for tissue banking, including comprehensive donor screening and extensive microbiological testing.”
Source: Bioventus

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







