Copy to clipboard
Copy to clipboard 
Bioretec received FDA 510(k) clearance to market its bioresorbable RemeOs trauma screw. Following this market authorization, Bioretec has resolved to update and refocus the company’s product portfolio to facilitate an accelerated commercialization strategy and provide a more synergistic offering to the market. Further, Bioretec has refined its U.S. go-to-market strategy to fit the company’s resources better and provide enhanced possibilities to penetrate the U.S. market. Financial targets are updated to reflect these strategic changes.
Additionally, Bioretec is also exploring possibilities to raise additional financing to accelerate the U.S. commercialization of RemeOs products, increasing manufacturing capacity and enhancing product development.
Bioretec will focus on serving customers in the lower and upper extremities segments with products that have a faster commercialization strategy and provide a more synergistic offering. To complement the trauma screw product group, Bioretec will add two new product groups, RemeOs staples and RemeOs plates, to its product development pipeline.
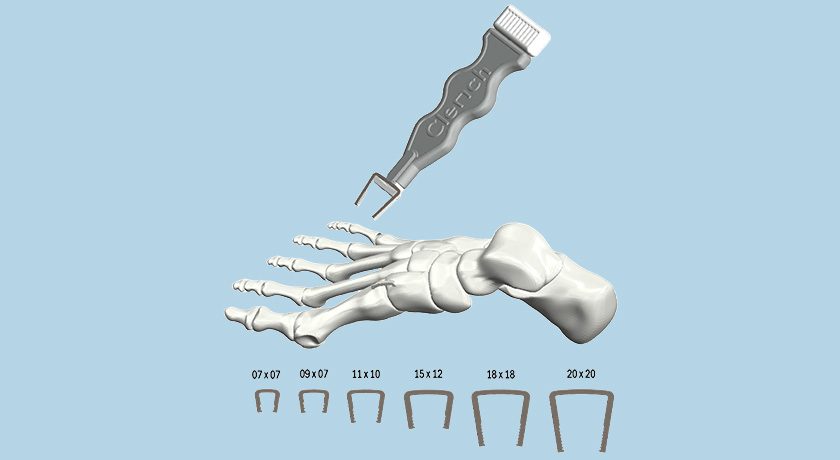
RemeOs staples are used individually or with RemeOs trauma screws to stabilize a fracture or osteotomy, and are used mainly in the foot and ankle area, whereas RemeOs plates are used for similar indications when additional support is needed, and further to be used together with RemeOs trauma screws if multiple fractures have occurred in the same anatomical location.
RemeOs staples are expected to enter commercialization during 2026. RemeOs plates are expected to follow RemeOs staples and enter commercialization during 2027. Additionally, RemeOs products will also be developed for pediatric use.
Due to the expansion of the synergistic product portfolio, the commercialization of RemeOs DrillPin is transferred to 2025, and an IM-nail and spinal cage are transferred to be launched after 2028, instead of earlier announced estimates of 2024, 2026 and 2027, respectively.
Source: Bioretec
Bioretec received FDA 510(k) clearance to market its bioresorbable RemeOs trauma screw. Following this market authorization, Bioretec has resolved to update and refocus the company’s product portfolio to facilitate an accelerated commercialization strategy and provide a more synergistic offering to the market. Further, Bioretec has refined...
Bioretec received FDA 510(k) clearance to market its bioresorbable RemeOs trauma screw. Following this market authorization, Bioretec has resolved to update and refocus the company’s product portfolio to facilitate an accelerated commercialization strategy and provide a more synergistic offering to the market. Further, Bioretec has refined its U.S. go-to-market strategy to fit the company’s resources better and provide enhanced possibilities to penetrate the U.S. market. Financial targets are updated to reflect these strategic changes.
Additionally, Bioretec is also exploring possibilities to raise additional financing to accelerate the U.S. commercialization of RemeOs products, increasing manufacturing capacity and enhancing product development.
Bioretec will focus on serving customers in the lower and upper extremities segments with products that have a faster commercialization strategy and provide a more synergistic offering. To complement the trauma screw product group, Bioretec will add two new product groups, RemeOs staples and RemeOs plates, to its product development pipeline.
RemeOs staples are used individually or with RemeOs trauma screws to stabilize a fracture or osteotomy, and are used mainly in the foot and ankle area, whereas RemeOs plates are used for similar indications when additional support is needed, and further to be used together with RemeOs trauma screws if multiple fractures have occurred in the same anatomical location.
RemeOs staples are expected to enter commercialization during 2026. RemeOs plates are expected to follow RemeOs staples and enter commercialization during 2027. Additionally, RemeOs products will also be developed for pediatric use.
Due to the expansion of the synergistic product portfolio, the commercialization of RemeOs DrillPin is transferred to 2025, and an IM-nail and spinal cage are transferred to be launched after 2028, instead of earlier announced estimates of 2024, 2026 and 2027, respectively.
Source: Bioretec

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.