
 Copy to clipboard
Copy to clipboard 
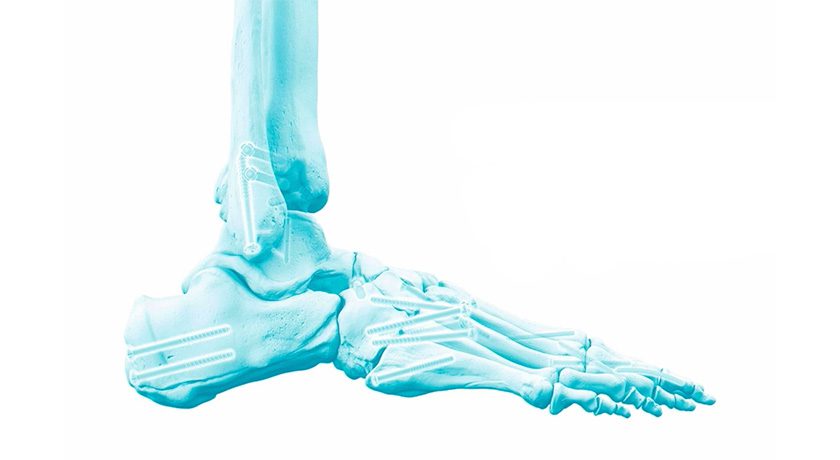
Bioretec completed its CE Mark approval process and can begin to market its RemeOs Trauma Screw portfolio within the European Union and non-European countries that recognize the CE Mark market authorization. This approval covers all cannulated and non-cannulated product designs (RemeOs FT cannulated, RemeOs FL cannulated, RemeOs FC cannulated and RemeOs LAG Solid), with sizes ranging from diameters of 2.0mm to 4.0mm and lengths from 8mm to 50mm. Indications approved include the use of these screws for fracture and malalignment fixations in both upper and lower extremities of adult and pediatric patients, excluding the hand and forefoot.
“We are extremely pleased that the EU market approval includes all designs and a vast number of indications. We can now immediately begin offering all RemeOs Trauma Screws to patients across Europe. Moreover, this approval paves the way for market entry into non-European countries that recognize the CE mark, and it empowers us to collect real-world clinical evidence. This evidence will enable the expansion of indications in the U.S., where the current approval is more limited,” comments Alan Donze, CEO of Bioretec.
Source: Bioretec
Bioretec completed its CE Mark approval process and can begin to market its RemeOs Trauma Screw portfolio within the European Union and non-European countries that recognize the CE Mark market authorization. This approval covers all cannulated and non-cannulated product designs (RemeOs FT cannulated, RemeOs FL cannulated, RemeOs FC cannulated and...
Bioretec completed its CE Mark approval process and can begin to market its RemeOs Trauma Screw portfolio within the European Union and non-European countries that recognize the CE Mark market authorization. This approval covers all cannulated and non-cannulated product designs (RemeOs FT cannulated, RemeOs FL cannulated, RemeOs FC cannulated and RemeOs LAG Solid), with sizes ranging from diameters of 2.0mm to 4.0mm and lengths from 8mm to 50mm. Indications approved include the use of these screws for fracture and malalignment fixations in both upper and lower extremities of adult and pediatric patients, excluding the hand and forefoot.
“We are extremely pleased that the EU market approval includes all designs and a vast number of indications. We can now immediately begin offering all RemeOs Trauma Screws to patients across Europe. Moreover, this approval paves the way for market entry into non-European countries that recognize the CE mark, and it empowers us to collect real-world clinical evidence. This evidence will enable the expansion of indications in the U.S., where the current approval is more limited,” comments Alan Donze, CEO of Bioretec.
Source: Bioretec

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







