
 Copy to clipboard
Copy to clipboard 
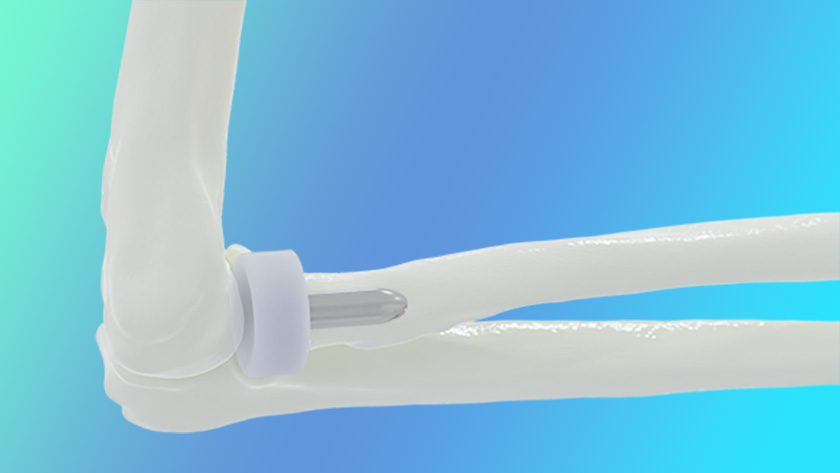
BioPoly received FDA 510(k) clearance to market the BioPoly Radial Head System for the elbow. This implant is manufactured from BioPoly’s proprietary material and is reported to be the only synthetic cartilage Radial Head on the market.
With this FDA clearance, BioPoly has taken significant steps toward achieving its strategy of expansion of its orthopedic, resurfacing implants in the US. The team has been actively developing multiple implant systems for many years as we worked toward gaining FDA clearance in the US market. Dr. Herb Schwartz, BioPoly’s Chairman and CTO, said, “We now have three FDA-cleared products in three different joints and we also have an FDA Master File for the material. The BioPoly Radial Head clearance shows that our synthetic cartilage platform has been accepted by FDA and the market. We have many more implant systems that are at various stages within the regulatory approval process; so, stay tuned as we continue to expand our implant offerings.”
“FDA clearance of the BioPoly Radial Head is a major accomplishment for our company, and I am very proud of our team which consists of surgeons, engineers, scientists and operations experts. It takes the entire team to develop an implant of this caliber which will change the landscape of radial head surgery because surgeons now have a synthetic cartilage option for their patients,” said Ryan Schlotterback, BioPoly’s President & CEO, “Since Radial Head implants articulate with patients’ natural cartilage, BioPoly is perfect for this application. We will be launching the system very soon. The surgeon team who helped develop this system and surgeons who have already been trained are very excited to have the BioPoly Radial Head available to them.”
The BioPoly material has been used clinically in multiple joints (knee, patella, trochlear groove, great toe and lesser toes) for over 12 years and has repeatedly demonstrated that it does not damage the opposing cartilage surface like metal implants.
Source: BioPoly LLC
BioPoly received FDA 510(k) clearance to market the BioPoly Radial Head System for the elbow. This implant is manufactured from BioPoly’s proprietary material and is reported to be the only synthetic cartilage Radial Head on the market.
With this FDA clearance, BioPoly has taken significant steps toward achieving its strategy of expansion of...
BioPoly received FDA 510(k) clearance to market the BioPoly Radial Head System for the elbow. This implant is manufactured from BioPoly’s proprietary material and is reported to be the only synthetic cartilage Radial Head on the market.
With this FDA clearance, BioPoly has taken significant steps toward achieving its strategy of expansion of its orthopedic, resurfacing implants in the US. The team has been actively developing multiple implant systems for many years as we worked toward gaining FDA clearance in the US market. Dr. Herb Schwartz, BioPoly’s Chairman and CTO, said, “We now have three FDA-cleared products in three different joints and we also have an FDA Master File for the material. The BioPoly Radial Head clearance shows that our synthetic cartilage platform has been accepted by FDA and the market. We have many more implant systems that are at various stages within the regulatory approval process; so, stay tuned as we continue to expand our implant offerings.”
“FDA clearance of the BioPoly Radial Head is a major accomplishment for our company, and I am very proud of our team which consists of surgeons, engineers, scientists and operations experts. It takes the entire team to develop an implant of this caliber which will change the landscape of radial head surgery because surgeons now have a synthetic cartilage option for their patients,” said Ryan Schlotterback, BioPoly’s President & CEO, “Since Radial Head implants articulate with patients’ natural cartilage, BioPoly is perfect for this application. We will be launching the system very soon. The surgeon team who helped develop this system and surgeons who have already been trained are very excited to have the BioPoly Radial Head available to them.”
The BioPoly material has been used clinically in multiple joints (knee, patella, trochlear groove, great toe and lesser toes) for over 12 years and has repeatedly demonstrated that it does not damage the opposing cartilage surface like metal implants.
Source: BioPoly LLC

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







