
 Copy to clipboard
Copy to clipboard 
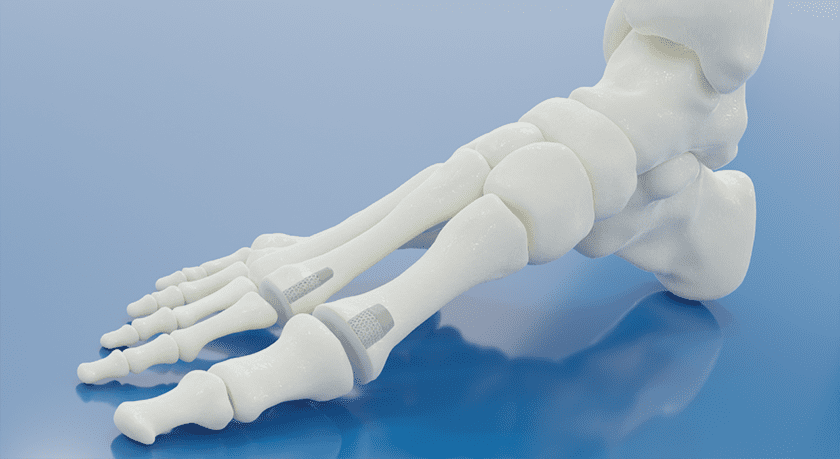
BioPoly received FDA 510(k) clearance to market its BioPoly® Lesser Toe System, BioPoly’s second FDA-cleared product in the U.S. market.
BioPoly’s Great Toe System was its first product to receive FDA clearance.
BioPoly biomaterial is a combination of polyethylene (UHMWPE) and hyaluronic acid. This proprietary combination results in a strong, hydrophilic material that behaves like a synthetic cartilage with the ability to articulate with native cartilage while minimizing damage to the native tissue. The BioPoly Lesser Toe implant replaces one side of the joint with a cartilage-friendly surface and robust fixation to the bone, maintaining motion at the joint while allowing the patient’s healthy cartilage to remain intact.
“We are excited about achieving our second FDA clearance with BioPoly technology as we continue to build momentum in our extremity portfolio. The Great Toe System is performing well clinically and we expect the same from our BioPoly Lesser Toe System,” said Ryan Schlotterback, BioPoly President and CEO. “We plan to launch the Lesser Toe System into the market mid-2023. In addition, we have some exciting products in the upper extremity that we anticipate announcing in the new year.”
According to Sheila Schwartz, BioPoly COO, “Our manufacturing and supply channels are prepared to handle the increasing demand for our BioPoly products. Therefore, as we introduce the BioPoly Lesser Toe System along with the BioPoly Great Toe System, we have ample capacity with room for growth as we add even more implant product lines which are already in the pipeline.”
Source: BioPoly LLC
BioPoly received FDA 510(k) clearance to market its BioPoly® Lesser Toe System, BioPoly’s second FDA-cleared product in the U.S. market.
BioPoly’s Great Toe System was its first product to receive FDA clearance.
BioPoly biomaterial is a combination of polyethylene (UHMWPE) and hyaluronic acid. This proprietary combination results in a...
BioPoly received FDA 510(k) clearance to market its BioPoly® Lesser Toe System, BioPoly’s second FDA-cleared product in the U.S. market.
BioPoly’s Great Toe System was its first product to receive FDA clearance.
BioPoly biomaterial is a combination of polyethylene (UHMWPE) and hyaluronic acid. This proprietary combination results in a strong, hydrophilic material that behaves like a synthetic cartilage with the ability to articulate with native cartilage while minimizing damage to the native tissue. The BioPoly Lesser Toe implant replaces one side of the joint with a cartilage-friendly surface and robust fixation to the bone, maintaining motion at the joint while allowing the patient’s healthy cartilage to remain intact.
“We are excited about achieving our second FDA clearance with BioPoly technology as we continue to build momentum in our extremity portfolio. The Great Toe System is performing well clinically and we expect the same from our BioPoly Lesser Toe System,” said Ryan Schlotterback, BioPoly President and CEO. “We plan to launch the Lesser Toe System into the market mid-2023. In addition, we have some exciting products in the upper extremity that we anticipate announcing in the new year.”
According to Sheila Schwartz, BioPoly COO, “Our manufacturing and supply channels are prepared to handle the increasing demand for our BioPoly products. Therefore, as we introduce the BioPoly Lesser Toe System along with the BioPoly Great Toe System, we have ample capacity with room for growth as we add even more implant product lines which are already in the pipeline.”
Source: BioPoly LLC

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







