
 Copy to clipboard
Copy to clipboard 
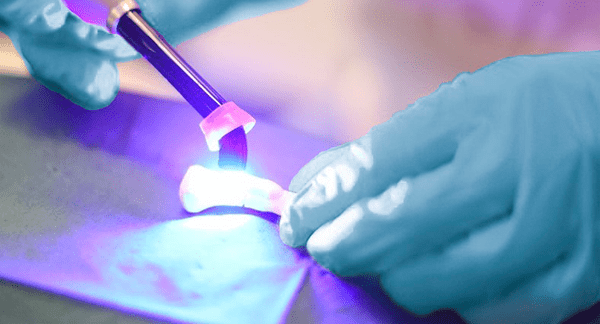
Biomedical Bonding (BMB) secured an additional patent in China based on a unique primer formulation for use together with its resin-based composite technology directly on wet bone substrates or metal implants.
The patent in China is a step toward the commercialization of BMB’s Bonevolent™ line of products for fracture fixation. Bonevolent technology is customizable by the surgeon and enables patient-specific adaptation to the fracture and bone geometry. The technology is also biocompatible and does not cause irritation or soft tissue adhesion.
This represents the company’s second granted patent in China.
“BMB continues to expand its Bonevolent™ IPR portfolio in key geographical positions…China is considered as an attractive market for the company’s fracture fixation products. With this patent approved, the IPR for our primer technology is further strengthened as it protects all key components facilitating exceptional resins-based fixation of bones, metal or teeth. As a company, such approval is a crucial element and allows BMB to pursue the commercialization of its composite-based fixators towards patient-customized treatment of both bone fractures as well dental restorations,” said Founder and CEO, Prof. Michael Malkoch.
Source: Biomedical Bonding
Biomedical Bonding (BMB) secured an additional patent in China based on a unique primer formulation for use together with its resin-based composite technology directly on wet bone substrates or metal implants.
The patent in China is a step toward the commercialization of BMB’s Bonevolent™ line of products for fracture fixation. Bonevolent...
Biomedical Bonding (BMB) secured an additional patent in China based on a unique primer formulation for use together with its resin-based composite technology directly on wet bone substrates or metal implants.
The patent in China is a step toward the commercialization of BMB’s Bonevolent™ line of products for fracture fixation. Bonevolent technology is customizable by the surgeon and enables patient-specific adaptation to the fracture and bone geometry. The technology is also biocompatible and does not cause irritation or soft tissue adhesion.
This represents the company’s second granted patent in China.
“BMB continues to expand its Bonevolent™ IPR portfolio in key geographical positions…China is considered as an attractive market for the company’s fracture fixation products. With this patent approved, the IPR for our primer technology is further strengthened as it protects all key components facilitating exceptional resins-based fixation of bones, metal or teeth. As a company, such approval is a crucial element and allows BMB to pursue the commercialization of its composite-based fixators towards patient-customized treatment of both bone fractures as well dental restorations,” said Founder and CEO, Prof. Michael Malkoch.
Source: Biomedical Bonding

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







