
 Copy to clipboard
Copy to clipboard 
Bio2 Technologies enrolled the first patient in the randomized, multi-center, non-inferiority Investigational Device Exemption clinical trial of the Vitrium cervical interbody device. The 168-patient study will compare Vitrium to allograft in spinal fusion.
The company received FDA approval for the trial in 4Q18 after pre-clinical studies demonstrated that Vitrium bioactive glass implants are substituted with newly-regenerated bone while exhibiting sufficient strength for use in load-bearing reconstructive surgeries.
Vitrium is a proprietary structural and resorbable orthobiomaterial that uses the patient’s own regenerative process to achieve fusion without metal or plastic left behind.
Tom Cha, M.D., Principal Investigator of the Bio2 study, remarked, “The Vitrium material is a fundamental improvement over the current solutions for spinal fusion. The implant uses a clinically proven bioactive material in a form that offers optimal strength and porosity to facilitate bone remodeling throughout the fusion site.”
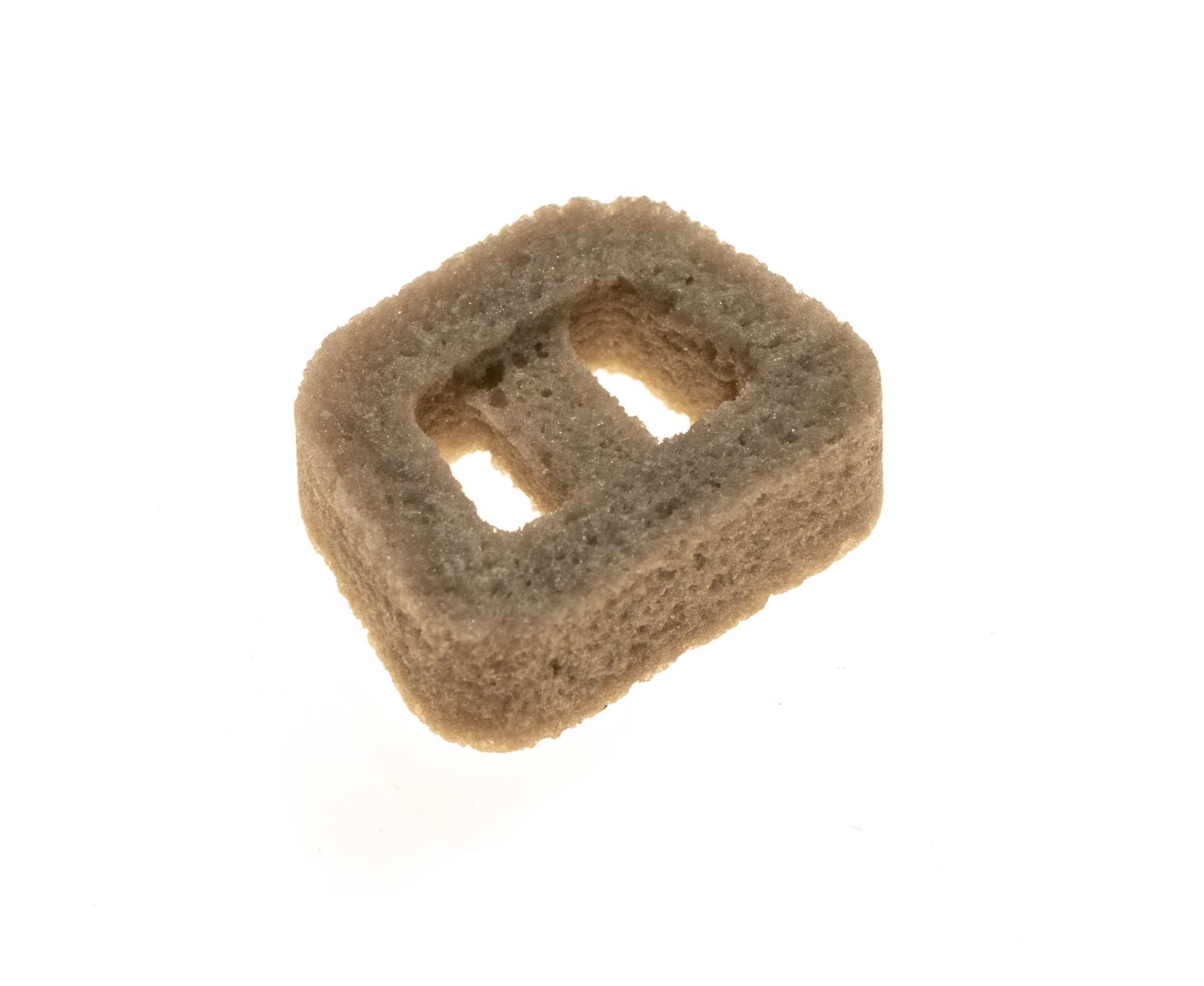
Source: Bio2 Technologies, Inc.
Bio2 Technologies enrolled the first patient in the randomized, multi-center, non-inferiority Investigational Device Exemption clinical trial of the Vitrium cervical interbody device. The 168-patient study will compare Vitrium to allograft in spinal fusion.
The company received FDA approval for the trial in 4Q18 after pre-clinical studies...
Bio2 Technologies enrolled the first patient in the randomized, multi-center, non-inferiority Investigational Device Exemption clinical trial of the Vitrium cervical interbody device. The 168-patient study will compare Vitrium to allograft in spinal fusion.
The company received FDA approval for the trial in 4Q18 after pre-clinical studies demonstrated that Vitrium bioactive glass implants are substituted with newly-regenerated bone while exhibiting sufficient strength for use in load-bearing reconstructive surgeries.
Vitrium is a proprietary structural and resorbable orthobiomaterial that uses the patient’s own regenerative process to achieve fusion without metal or plastic left behind.
Tom Cha, M.D., Principal Investigator of the Bio2 study, remarked, “The Vitrium material is a fundamental improvement over the current solutions for spinal fusion. The implant uses a clinically proven bioactive material in a form that offers optimal strength and porosity to facilitate bone remodeling throughout the fusion site.”

Source: Bio2 Technologies, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







