
 Copy to clipboard
Copy to clipboard 
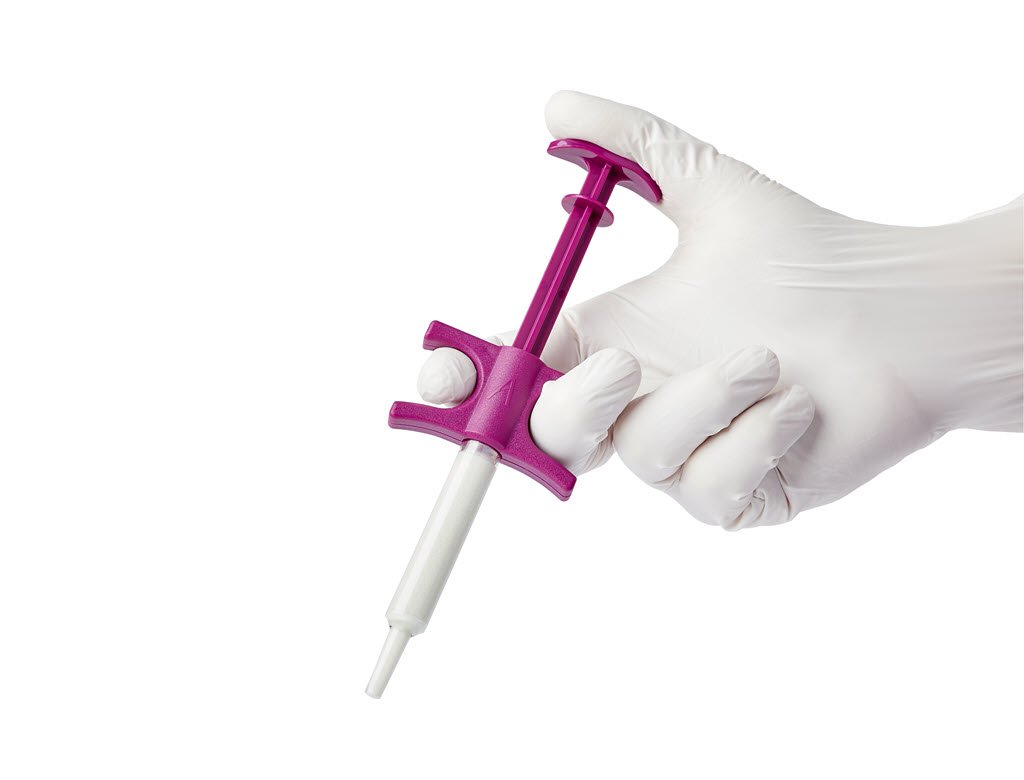 Baxter International was granted FDA 510(k) clearance to market Actifuse Flow bone graft substitute, offered in a prepackaged delivery syringe for precise placement into small bony voids or gaps.
Baxter International was granted FDA 510(k) clearance to market Actifuse Flow bone graft substitute, offered in a prepackaged delivery syringe for precise placement into small bony voids or gaps.
U.S. launch is expected by 4Q18, and Actifuse Flow will be sold in 1.5-, 3- and 5-mL sizes. The product has application in a variety of orthopaedic procedures in the pelvis, extremities and posterolateral spine.
Baxter acquired Actifuse from ApaTech in 2010. The company’s orthobiologic portfolio also includes Actifuse ABX, Actifuse Shape, Actifuse MIS and Altapore.
Sources: Baxter International; ORTHOWORLD, Inc.; Image courtesy of Baxter International
Baxter International was granted FDA 510(k) clearance to market Actifuse Flow bone graft substitute, offered in a prepackaged delivery syringe for precise placement into small bony voids or gaps.
U.S. launch is expected by 4Q18, and Actifuse Flow will be sold in 1.5-, 3- and 5-mL sizes. The product has application in a variety of orthopaedic...
 Baxter International was granted FDA 510(k) clearance to market Actifuse Flow bone graft substitute, offered in a prepackaged delivery syringe for precise placement into small bony voids or gaps.
Baxter International was granted FDA 510(k) clearance to market Actifuse Flow bone graft substitute, offered in a prepackaged delivery syringe for precise placement into small bony voids or gaps.
U.S. launch is expected by 4Q18, and Actifuse Flow will be sold in 1.5-, 3- and 5-mL sizes. The product has application in a variety of orthopaedic procedures in the pelvis, extremities and posterolateral spine.
Baxter acquired Actifuse from ApaTech in 2010. The company’s orthobiologic portfolio also includes Actifuse ABX, Actifuse Shape, Actifuse MIS and Altapore.
Sources: Baxter International; ORTHOWORLD, Inc.; Image courtesy of Baxter International

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







