
 Copy to clipboard
Copy to clipboard 
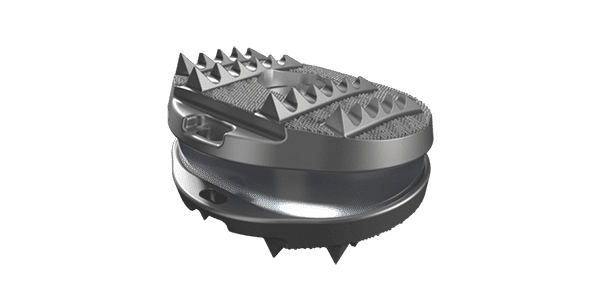
On August 8, 2022, AxioMed submitted to FDA all of the required documents for Module III, the final Module containing all of the data tables comparing AxioMed to Centinel Spine’s prodisc as the control. The final step for AxioMed to get U.S. market approval will be an FDA site inspection of the cleanroom manufacturing facilities in Massachusetts.
AxioMed has what is reported to be the only lumbar viscoelastic disc replacement medical device in the U.S. with global clinical use since 2008 and no device failure. Five-year followup data shows no revisions and rare cases of heterotopic bone formation. The company’s Module III lumbar clinical data also shows no production of wear debris.
Source: AxioMed
On August 8, 2022, AxioMed submitted to FDA all of the required documents for Module III, the final Module containing all of the data tables comparing AxioMed to Centinel Spine's prodisc as the control. The final step for AxioMed to get U.S. market approval will be an FDA site inspection of the cleanroom manufacturing facilities in...
On August 8, 2022, AxioMed submitted to FDA all of the required documents for Module III, the final Module containing all of the data tables comparing AxioMed to Centinel Spine’s prodisc as the control. The final step for AxioMed to get U.S. market approval will be an FDA site inspection of the cleanroom manufacturing facilities in Massachusetts.
AxioMed has what is reported to be the only lumbar viscoelastic disc replacement medical device in the U.S. with global clinical use since 2008 and no device failure. Five-year followup data shows no revisions and rare cases of heterotopic bone formation. The company’s Module III lumbar clinical data also shows no production of wear debris.
Source: AxioMed

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







