Copy to clipboard
Copy to clipboard 
Following closure of an oversubscribed first round, AxioMed opened a US $10MM round of funding to support operations and ex-U.S. sales.
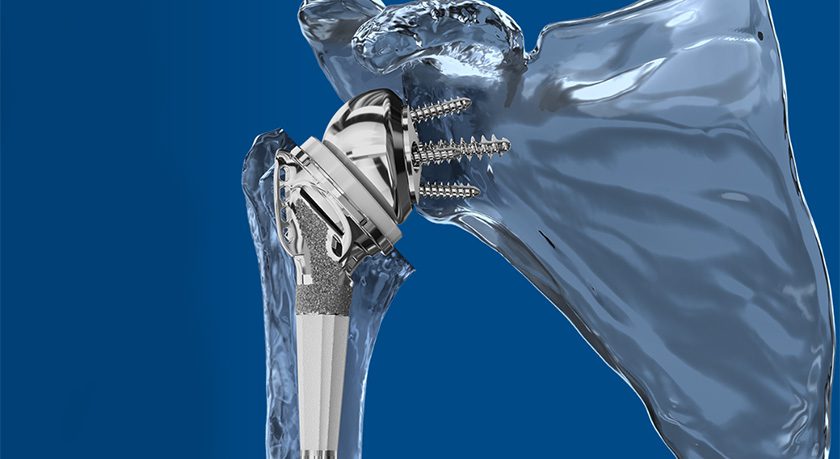
In 1Q17, the company completed collection and analysis of 2-year follow-up data in its U.S. Investigational Device Exemption (IDE) clinical study of its viscoelastic lumbar disc. AxioMed expects 1Q18 CE Mark approval for its lateral disc, and is pursuing a multi-level cervical IDE in the U.S.
Sources: AxioMed; ORTHOWORLD Inc.
Following closure of an oversubscribed first round, AxioMed opened a US $10MM round of funding to support operations and ex-U.S. sales.
In 1Q17, the company completed collection and analysis of 2-year follow-up data in its U.S. Investigational Device Exemption (IDE) clinical study of its viscoelastic lumbar disc....
Following closure of an oversubscribed first round, AxioMed opened a US $10MM round of funding to support operations and ex-U.S. sales.
In 1Q17, the company completed collection and analysis of 2-year follow-up data in its U.S. Investigational Device Exemption (IDE) clinical study of its viscoelastic lumbar disc. AxioMed expects 1Q18 CE Mark approval for its lateral disc, and is pursuing a multi-level cervical IDE in the U.S.
Sources: AxioMed; ORTHOWORLD Inc.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.