
 Copy to clipboard
Copy to clipboard 
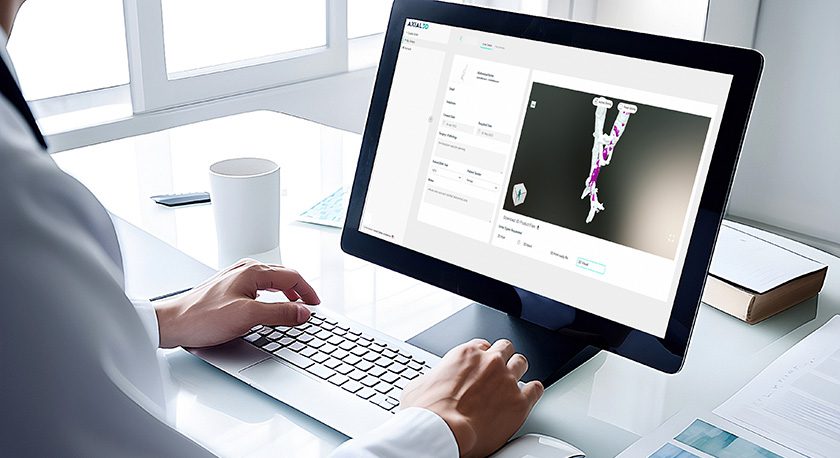
Axial3D received FDA 510(k) clearance to market an automated, artificial intelligence (AI)-driven, cloud-based segmentation platform for orthopedic trauma, orthopedic and maxillofacial applications. This is the second FDA clearance that Axial3D has received for its segmentation platform, INSIGHT.
Differentiated by its AWS cloud-based infrastructure, AI algorithms and advanced machine learning techniques, Axial3D INSIGHT automates the conversion process of 2D DICOM images such as CT and MRI scans into accurate 3D visualizations, 3D print-ready files, 3D mesh files or 3D printed anatomical models, made with Stratasys print technology. By automating the task of manual or semi-manual image segmentation, Axial3D enables healthcare providers and medical device companies to address more applications while saving hours or days per case and reducing capital expenditures.
The Axial INSIGHT platform also allows device companies to accelerate their patient-specific programs quickly by being able to process more patient data with the same resources. This 3D data can then be used to design personalized devices and surgical kits that include surgical plans, models, and surgical guides, that can be 3D printed on a variety of printers, including Stratasys systems from Digital Anatomy 3D printers to production-scale additive manufacturing systems.
“Our FDA clearance for Axial3D INSIGHT™ is a testament to how far Axial3D has come,” stated Dan Crawford, Founder and CSO of Axial3D. “From our humble beginnings as a startup to now being recognized as a leading medical technology company, this achievement showcases our dedication to pushing the boundaries of innovation in healthcare. We are immensely proud of our team’s commitment in delivering exceptional patient care using advanced automation, artificial intelligence, and machine learning technologies.”
Source: Axial3D
Axial3D received FDA 510(k) clearance to market an automated, artificial intelligence (AI)-driven, cloud-based segmentation platform for orthopedic trauma, orthopedic and maxillofacial applications. This is the second FDA clearance that Axial3D has received for its segmentation platform, INSIGHT.
Differentiated by its AWS cloud-based...
Axial3D received FDA 510(k) clearance to market an automated, artificial intelligence (AI)-driven, cloud-based segmentation platform for orthopedic trauma, orthopedic and maxillofacial applications. This is the second FDA clearance that Axial3D has received for its segmentation platform, INSIGHT.
Differentiated by its AWS cloud-based infrastructure, AI algorithms and advanced machine learning techniques, Axial3D INSIGHT automates the conversion process of 2D DICOM images such as CT and MRI scans into accurate 3D visualizations, 3D print-ready files, 3D mesh files or 3D printed anatomical models, made with Stratasys print technology. By automating the task of manual or semi-manual image segmentation, Axial3D enables healthcare providers and medical device companies to address more applications while saving hours or days per case and reducing capital expenditures.
The Axial INSIGHT platform also allows device companies to accelerate their patient-specific programs quickly by being able to process more patient data with the same resources. This 3D data can then be used to design personalized devices and surgical kits that include surgical plans, models, and surgical guides, that can be 3D printed on a variety of printers, including Stratasys systems from Digital Anatomy 3D printers to production-scale additive manufacturing systems.
“Our FDA clearance for Axial3D INSIGHT™ is a testament to how far Axial3D has come,” stated Dan Crawford, Founder and CSO of Axial3D. “From our humble beginnings as a startup to now being recognized as a leading medical technology company, this achievement showcases our dedication to pushing the boundaries of innovation in healthcare. We are immensely proud of our team’s commitment in delivering exceptional patient care using advanced automation, artificial intelligence, and machine learning technologies.”
Source: Axial3D

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







