
 Copy to clipboard
Copy to clipboard 
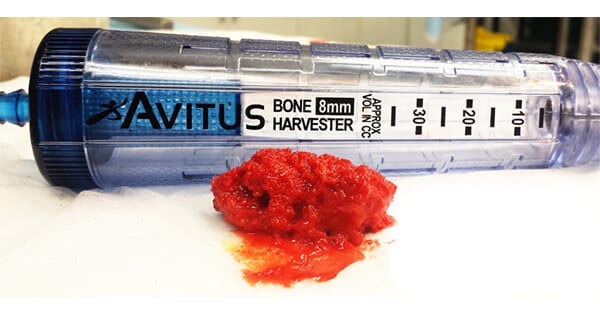
Avitus Orthopaedics launched the Avitus® Bone Harvester – 6mm Edition for harvesting small to large volumes of autologous bone graft and marrow from smaller bone anatomies, such as the calcaneus and distal radius. The new device features a 30% smaller pilot hole entry than the standard edition.
In 2015, the standard edition was granted FDA 510(k) clearance as a Class II medical device, indicated to harvest autologous bone graft and bone marrow in a minimally invasive manner for use in spine and orthopaedic procedures.
“We developed the original Avitus Bone Harvester technology to solve an important clinical problem – surgeons needed a simple way to obtain high quality autograft and marrow minimally invasively for their patients,” said Maxim Budyansky, Co-founder and Chief Technology Officer of Avitus. “…The 6mm edition builds on our offering, providing surgeons with the versatility to harvest from various anatomies for their streamlined bone and marrow harvests.”
Steven Lotz, Avitus Vice President of Sales, said, “Our rapidly growing network of distribution partners find Avitus to be a powerful tool in their sales bag. It offers them a unique and truly value-additive product to put in front of their surgeons, who are galvanized by the opportunity to provide their patients with gold standard autologous graft and marrow while delivering significant cost savings to their hospitals.”
Avitus Orthopaedics launched the Avitus® Bone Harvester - 6mm Edition for harvesting small to large volumes of autologous bone graft and marrow from smaller bone anatomies, such as the calcaneus and distal radius. The new device features a 30% smaller pilot hole entry than the standard edition.
In 2015, the standard edition was granted...
Avitus Orthopaedics launched the Avitus® Bone Harvester – 6mm Edition for harvesting small to large volumes of autologous bone graft and marrow from smaller bone anatomies, such as the calcaneus and distal radius. The new device features a 30% smaller pilot hole entry than the standard edition.
In 2015, the standard edition was granted FDA 510(k) clearance as a Class II medical device, indicated to harvest autologous bone graft and bone marrow in a minimally invasive manner for use in spine and orthopaedic procedures.
“We developed the original Avitus Bone Harvester technology to solve an important clinical problem – surgeons needed a simple way to obtain high quality autograft and marrow minimally invasively for their patients,” said Maxim Budyansky, Co-founder and Chief Technology Officer of Avitus. “…The 6mm edition builds on our offering, providing surgeons with the versatility to harvest from various anatomies for their streamlined bone and marrow harvests.”
Steven Lotz, Avitus Vice President of Sales, said, “Our rapidly growing network of distribution partners find Avitus to be a powerful tool in their sales bag. It offers them a unique and truly value-additive product to put in front of their surgeons, who are galvanized by the opportunity to provide their patients with gold standard autologous graft and marrow while delivering significant cost savings to their hospitals.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







