
 Copy to clipboard
Copy to clipboard 
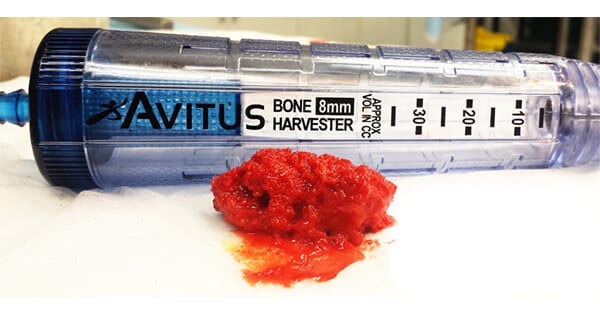
Avitus Orthopaedics received FDA clearance to market expanded indications for use of its Avitus® Bone Harvester. This clearance allows for the use of the technology to debride and capture infected, necrotic or diseased cancellous bone, such as in the removal of osteomyelitis and tumors.
The Avitus Bone Harvester is a suction-powered instrument used to harvest autologous bone graft and liquid marrow for a range of orthopedic surgeries such as fusions, reconstructions and fracture non-unions. Avitus comes in multiple sizes, providing the flexibility to harvest from various anatomies.
Avitus Orthopaedics received initial FDA clearance and launched the Avitus Bone Harvester in 2016.
“In applications for the removal of infected or diseased bone, the Avitus Bone Harvester offers several key advantages,” said Maxim Budyansky, Avitus Co-Founder and Chief Technology Officer. “The patented suction-powered technology enables the removal of diseased bone quickly and minimally invasively. The collected tissue is contained within the handle of the device, minimizing exposure to surrounding healthy tissue and allowing for easy transport of the diseased tissue out of the surgical field without contamination of other instruments and the surgical site. The tissue can be discarded, or it can be simply retrieved from the device if subsequent pathology analysis is required.”
Avitus Orthopaedics received FDA clearance to market expanded indications for use of its Avitus® Bone Harvester. This clearance allows for the use of the technology to debride and capture infected, necrotic or diseased cancellous bone, such as in the removal of osteomyelitis and tumors.
The Avitus Bone Harvester is a suction-powered...
Avitus Orthopaedics received FDA clearance to market expanded indications for use of its Avitus® Bone Harvester. This clearance allows for the use of the technology to debride and capture infected, necrotic or diseased cancellous bone, such as in the removal of osteomyelitis and tumors.
The Avitus Bone Harvester is a suction-powered instrument used to harvest autologous bone graft and liquid marrow for a range of orthopedic surgeries such as fusions, reconstructions and fracture non-unions. Avitus comes in multiple sizes, providing the flexibility to harvest from various anatomies.
Avitus Orthopaedics received initial FDA clearance and launched the Avitus Bone Harvester in 2016.
“In applications for the removal of infected or diseased bone, the Avitus Bone Harvester offers several key advantages,” said Maxim Budyansky, Avitus Co-Founder and Chief Technology Officer. “The patented suction-powered technology enables the removal of diseased bone quickly and minimally invasively. The collected tissue is contained within the handle of the device, minimizing exposure to surrounding healthy tissue and allowing for easy transport of the diseased tissue out of the surgical field without contamination of other instruments and the surgical site. The tissue can be discarded, or it can be simply retrieved from the device if subsequent pathology analysis is required.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







