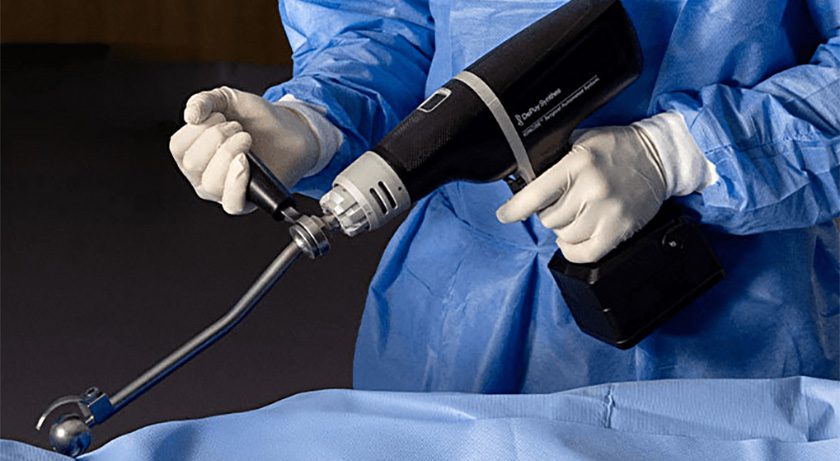
Arthrex Launches Synergy Power Instrument Suite
Synergy Power represents a versatile and reliable battery-powered system designed for a variety of orthopedic applications, including sports, arthroplasty, trauma and distal extremities procedures. Jun 04, 2025