
 Copy to clipboard
Copy to clipboard 
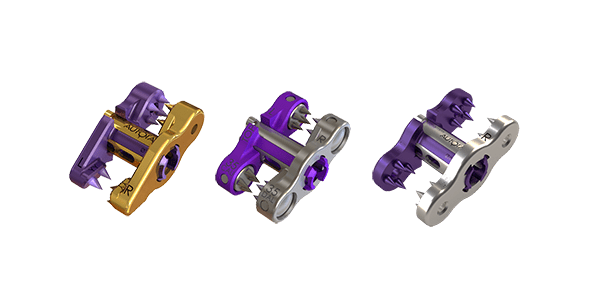
Aurora Spine announced FDA clearance of a new lumbar spinal stenosis indication for its ZIP™ family of MIS implants.
The ZIP series features various bone anchors, Aurora Spine’s patented one-step locking mechanism with no set screw and a large graft space designed for biologic materials. The ZIP product line is Aurora Spine’s minimally invasive interlaminar fixation implant for spinal fusion and was developed as an alternative to pedicle screw fixation.
The Aurora Spine ZIP MIS Interspinous Fusion System is a posterior, non-pedicle supplemental fixation device, intended for use in the non-cervical spine. It is intended for plate fixation/attachment to the spinous process for the purpose of achieving supplemental fusion in the following conditions: degenerative disc disease (defined as back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies), lumbar spinal stenosis, spondylolisthesis, trauma (i.e., fracture or dislocation) and/or tumor. The Aurora Spine ZIP MIS Interspinous Fusion System is intended for use with bone graft material and is not intended for stand-alone use.
“The receipt of the additional Lumbar Spinal Stenosis indication for our ZIP MIS Interspinous Fusion System is another key milestone for Aurora Spine and allows us to expand our spinal product portfolio,” said Trent J. Northcutt, President, and Chief Executive Officer of Aurora Spine.
Source: Aurora Spine
Aurora Spine announced FDA clearance of a new lumbar spinal stenosis indication for its ZIP™ family of MIS implants.
The ZIP series features various bone anchors, Aurora Spine's patented one-step locking mechanism with no set screw and a large graft space designed for biologic materials. The ZIP product line is Aurora Spine's minimally invasive...
Aurora Spine announced FDA clearance of a new lumbar spinal stenosis indication for its ZIP™ family of MIS implants.
The ZIP series features various bone anchors, Aurora Spine’s patented one-step locking mechanism with no set screw and a large graft space designed for biologic materials. The ZIP product line is Aurora Spine’s minimally invasive interlaminar fixation implant for spinal fusion and was developed as an alternative to pedicle screw fixation.
The Aurora Spine ZIP MIS Interspinous Fusion System is a posterior, non-pedicle supplemental fixation device, intended for use in the non-cervical spine. It is intended for plate fixation/attachment to the spinous process for the purpose of achieving supplemental fusion in the following conditions: degenerative disc disease (defined as back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies), lumbar spinal stenosis, spondylolisthesis, trauma (i.e., fracture or dislocation) and/or tumor. The Aurora Spine ZIP MIS Interspinous Fusion System is intended for use with bone graft material and is not intended for stand-alone use.
“The receipt of the additional Lumbar Spinal Stenosis indication for our ZIP MIS Interspinous Fusion System is another key milestone for Aurora Spine and allows us to expand our spinal product portfolio,” said Trent J. Northcutt, President, and Chief Executive Officer of Aurora Spine.
Source: Aurora Spine

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







